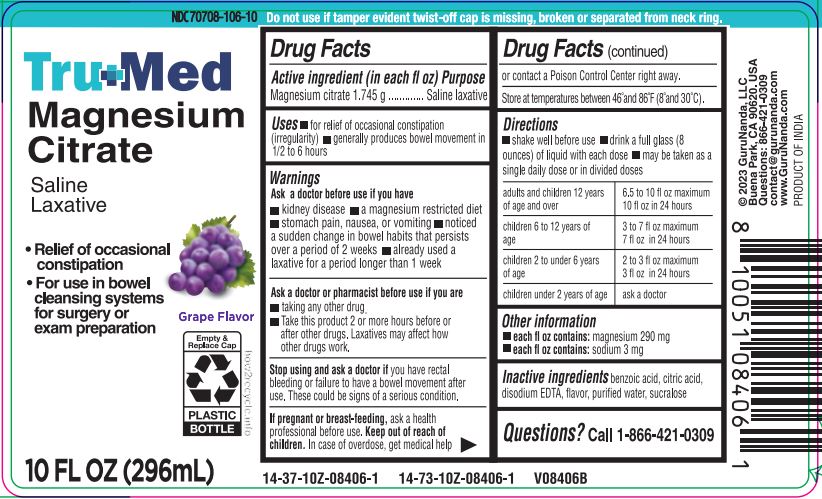
TrueMed Magnesium Citrate Saline Laxative- Grape
TrueMed by
Drug Labeling and Warnings
TrueMed by is a Otc medication manufactured, distributed, or labeled by GURUNANDA, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
TRUEMED SALINE LAXATIVE - GRAPE- magnesium citrate liquid
GURUNANDA, LLC
----------
TrueMed Magnesium Citrate Saline Laxative- Grape
Uses
- for relief of occasional constipation (irregularity)
- generally produces bowel movement in 1/2 to 6 hours
Warnings
Ask a docotor before use if you have
- kidney disease
- a magnesium restricted diet
- a potassium restricted diet
- stomach pain, nausea, or vomiting
- noticed a sudden change in bowel habits that persists over a period of 2 weeks
- already used a laxative for a period longer than 1 week
Ask a doctor or pharmacist before use if you are
- taking any other drug.
- Take this product 2 or more hours before or after other drugs. Laxatives may affect how other drug work.
Directions
- shake well before use
- drink a full glass (8 ounces) of liquid with each dose
- may be taken as a single daily dose or divided doses
| adults and children 12 years of age and over | 6.5 to 10 fl. oz., maximum 10 fl. oz. in 24 hours |
| children 6 to 12 years of age | 3 to 7 fl. oz., maximum 7 fl. oz. in 24 hours |
| children 2 to under 6 years of age | 2 to 3 fl. oz., maximum 3 fl. oz. in 24 hours |
| children under 2 years of age | ask a doctor |
| TRUEMED
SALINE LAXATIVE - GRAPE
magnesium citrate liquid |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - GURUNANDA, LLC (079671169) |
Revised: 11/2025
Document Id: 4346ffae-8bfa-8422-e063-6294a90af559
Set id: f888fdac-3649-23fa-e053-6394a90a9e86
Version: 10
Effective Time: 20251110
Trademark Results [TrueMed]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 TRUEMED 97831519 not registered Live/Pending |
True Medicine Inc. 2023-03-09 |
 TRUEMED 97596941 not registered Live/Pending |
Truemed Pharma Inc. 2022-09-19 |
 TRUEMED 88294275 not registered Live/Pending |
Structure Health & Wellness, LTD. 2019-02-08 |
 TRUEMED 86848811 not registered Dead/Abandoned |
Well And Pure, Inc 2015-12-14 |
 TRUEMED 79265887 not registered Live/Pending |
HeyDay Oy 2019-03-28 |
 TRUEMED 79241962 not registered Live/Pending |
HeyDay Oy 2018-08-17 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.