PARAPLATIN- carboplatin injection
PARAPLATIN by
Drug Labeling and Warnings
PARAPLATIN by is a Prescription medication manufactured, distributed, or labeled by Accord BioPharma Inc., Accord Healthcare Inc., Intas Pharmaceuticals Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
BOXED WARNING
(What is this?)
Paraplatin ® (carboplatin) Injection should be administered under the supervision of a qualified physician experienced in the use of cancer chemotherapeutic agents. Appropriate management of therapy and complications is possible only when adequate treatment facilities are readily available.
Bone marrow suppression is dose related and may be severe, resulting in infection and/or bleeding. Anemia may be cumulative and may require transfusion support. Vomiting is another frequent drug related side effect.
Anaphylactic-like reactions to carboplatin have been reported and may occur within minutes of Paraplatin ® administration. Epinephrine, corticosteroids, and antihistamines have been employed to alleviate symptoms.
-
DESCRIPTION
Paraplatin ® injection is supplied as a sterile, pyrogen-free, 10 mg/mL aqueous solution of carboplatin, USP. Carboplatin, USP is a platinum coordination compound. The chemical name for carboplatin, USP is platinum, diammine [1,1-cyclobutanedicarboxylato(2-)- O,O']-,(SP-4-2), and carboplatin, USP has the following structural formula:
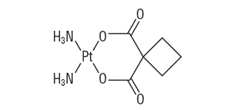
Carboplatin, USP is a crystalline powder. It is soluble in water at a rate of approximately 14 mg/mL, and the pH of a 1% solution is 5 to 7. It is virtually insoluble in ethanol, acetone, and dimethylacetamide.
-
CLINICAL PHARMACOLOGY
Carboplatin, like cisplatin, produces predominantly interstrand DNA cross-links rather than DNA-protein cross-links. This effect is apparently cell-cycle nonspecific. The aquation of carboplatin, which is thought to produce the active species, occurs at a slower rate than in the case of cisplatin. Despite this difference, it appears that both carboplatin and cisplatin induce equal numbers of drug-DNA cross-links, causing equivalent lesions and biological effects. The differences in potencies for carboplatin and cisplatin appear to be directly related to the difference in aquation rates.
In patients with creatinine clearances of about 60 mL/min or greater, plasma levels of intact carboplatin decay in a biphasic manner after a 30-minute intravenous infusion of 300 mg/m 2 to 500 mg/m 2 of carboplatin. The initial plasma half-life (alpha) was found to be 1.1 to 2 hours (n=6), and the postdistribution plasma half-life (beta) was found to be 2.6 to 5.9 hours (n=6). The total body clearance, apparent volume of distribution and mean residence time for carboplatin are 4.4 L/hour, 16 L and 3.5 hours, respectively. The C max values and areas under the plasma concentration versus time curves from 0 to infinity (AUC inf) increase linearly with dose, although the increase was slightly more than dose proportional. Carboplatin, therefore, exhibits linear pharmacokinetics over the dosing range studied (300 mg/m 2 to 500 mg/m 2).
Carboplatin is not bound to plasma proteins. No significant quantities of protein-free, ultrafilterable platinum-containing species other than carboplatin are present in plasma. However, platinum from carboplatin becomes irreversibly bound to plasma proteins and is slowly eliminated with a minimum half-life of 5 days.
The major route of elimination of carboplatin is renal excretion. Patients with creatinine clearances of approximately 60 mL/min or greater excrete 65% of the dose in the urine within 12 hours and 71% of the dose within 24 hours. All of the platinum in the 24-hour urine is present as carboplatin. Only 3% to 5% of the administered platinum is excreted in the urine between 24 and 96 hours. There are insufficient data to determine whether biliary excretion occurs.
In patients with creatinine clearances below 60 mL/min, the total body and renal clearances of carboplatin decrease as the creatinine clearance decreases. Carboplatin dosages should therefore be reduced in these patients (see DOSAGE AND ADMINISTRATION).
The primary determinant of Paraplatin ® clearance is glomerular filtration rate (GFR) and this parameter of renal function is often decreased in elderly patients. Dosing formulas incorporating estimates of GFR (see DOSAGE AND ADMINISTRATION) to provide predictable Paraplatin ® plasma AUCs should be used in elderly patients to minimize the risk of toxicity.
-
CLINICAL STUDIES
Use with Cyclophosphamide for Initial Treatment of Ovarian Cancer
In two prospectively randomized, controlled studies conducted by the National Cancer Institute of Canada, Clinical Trials Group (NCIC) and the Southwest Oncology Group (SWOG), 789 chemotherapy naive patients with advanced ovarian cancer were treated with carboplatin or cisplatin, both in combination with cyclophosphamide every 28 days for 6 courses before surgical reevaluation. The following results were obtained from both studies:
Comparative Efficacy
Overview of Pivotal Trials NCIC SWOG Number of patients randomized 447 342 Median age (years) 60 62 Dose of cisplatin 75 mg/m 2 100 mg/m 2 Dose of carboplatin 300 mg/m 2 300 mg/m 2 Dose of cyclophosphamide 600 mg/m 2 600 mg/m 2 Residual tumor < 2 cm (number of patients) 39% (174/447) 14% (49/342) Clinical Response in Measurable Disease Patients NCIC SWOG Carboplatin (number of patients) 60% (48/80) 58% (48/83) Cisplatin (number of patients) 58% (49/85) 43% (33/76) 95% CI of difference
(Carboplatin-Cisplatin)(-13.9%, 18.6%) (-2.3%, 31.1%) Pathologic Complete Response * NCIC SWOG - * 114 Carboplatin and 109 Cisplatin patients did not undergo second look surgery in NCIC study. 90 Carboplatin and 106 Cisplatin patients did not undergo second look surgery in SWOG study.
Carboplatin (number of patients) 11% (24/224) 10% (17/171) Cisplatin (number of patients) 15% (33/223) 10% (17/171) 95% CI of difference
(Carboplatin-Cisplatin)(-10.7%, 2.5%) (-6.9%, 6.9%) Progression-Free Survival (PFS) NCIC SWOG - * Kaplan-Meier Estimates Unrelated deaths occurring in the absence of progression were counted as events (progression) in this analysis.
- † Analysis adjusted for factors found to be of prognostic significance were consistent with unadjusted analysis.
Median Carboplatin 59 weeks 49 weeks Cisplatin 61 weeks 47 weeks 2-year PFS* Carboplatin 31% 21% Cisplatin 31% 21% 95% CI of difference
(Carboplatin-Cisplatin)(-9.3, 8.7) (-9.0, 9.4) 3-year PFS* Carboplatin 19% 8% Cisplatin 23% 14% 95% CI of difference
(Carboplatin-Cisplatin)(-11.5, 4.5) (-14.1, 0.3) Hazard Ratio† 1.10 1.02 95% CI
(Carboplatin-Cisplatin)(0.89, 1.35) (0.81, 1.29) Survival NCIC SWOG - * Kaplan-Meier Estimates
- † Analysis adjusted for factors found to be of prognostic significance were consistent with unadjusted analysis.
Median Carboplatin 110 weeks 86 weeks Cisplatin 99 weeks 79 weeks 2-year Survival* Carboplatin 51.9% 40.2% Cisplatin 48.4% 39.0% 95% CI of difference
(Carboplatin-Cisplatin)(-6.2, 13.2) (-9.8, 12.2) 3-year Survival* Carboplatin 34.6% 18.3% Cisplatin 33.1% 24.9% 95% CI of difference
(Carboplatin-Cisplatin)(-7.7, 10.7) (-15.9, 2.7) Hazard Ratio† 95% CI 0.98 1.01 (Carboplatin–Cisplatin) (0.78, 1.23) (0.78, 1.30) Comparative Toxicity
The pattern of toxicity exerted by the carboplatin-containing regimen was significantly different from that of the cisplatin-containing combinations. Differences between the two studies may be explained by different cisplatin dosages and by different supportive care.
The carboplatin-containing regimen induced significantly more thrombocytopenia and, in one study, significantly more leukopenia and more need for transfusional support. The cisplatin-containing regimen produced significantly more anemia in one study. However, no significant differences occurred in incidences of infections and hemorrhagic episodes
Non-hematologic toxicities (emesis, neurotoxicity, ototoxicity, renal toxicity, hypomagnesemia, and alopecia) were significantly more frequent in the cisplatin-containing arms.
ADVERSE EXPERIENCES IN PATIENTS WITH OVARIAN CANCER NCIC STUDY Carboplatin
Arm
Percent *Cisplatin Arm Percent * P-Values † - * Values are in percent of evaluable patients
- † ns=not significant, p>0.05
- ‡ May have been affected by cyclophosphamide dosage delivered
Bone Marrow Thrombocytopenia <100,000/mm 3 70 29 <0.001 <50,000/mm 3 41 6 <0.001 Neutropenia <2000 cells/mm 3 97 96 ns <1000 cells/mm 3 81 79 ns Leukopenia <4000 cells/mm 3 98 97 ns <2000 cells/mm 3 68 52 0.001 Anemia <11 g/dL 91 91 ns <8 g/dL 18 12 ns Infections 14 12 ns Bleeding 10 4 ns Transfusions 42 31 0.018 Gastrointestinal Nausea and vomiting 93 98 0.010 Vomiting 84 97 <0.001 Other GI side effects 50 62 0.013 Neurologic Peripheral neuropathies 16 42 <0.001 Ototoxicity 13 33 <0.001 Other sensory side effects 6 10 ns Central neurotoxicity 28 40 0.009 Renal Serum creatinine elevations 5 13 0.006 Blood urea elevations 17 31 <0.001 Hepatic Bilirubin elevations 5 3 ns SGOT elevations 17 13 ns Alkaline phosphatase elevations - - - Electrolytes loss Sodium 10 20 0.005 Potassium 16 22 ns Calcium 16 19 ns Magnesium 63 88 <0.001 Other side effects Pain 36 37 ns Asthenia 40 33 ns Cardiovascular 15 19 ns Respiratory 8 9 ns Allergic 12 9 ns Genitourinary 10 10 ns Alopecia ‡ 50 62 0.017 Mucositis 10 9 ns ADVERSE EXPERIENCES IN PATIENTS WITH OVARIAN CANCER SWOG STUDY Carboplatin Arm
Percent *Cisplatin Arm Percent * P-Values † - * Values are in percent of evaluable patients
- † ns=not significant, p>0.05
- ‡ May have been affected by cyclophosphamide dosage delivered
Bone Marrow Thrombocytopenia <100,000/mm 3 59 35 <0.001 <50,000/mm 3 22 11 0.006 Neutropenia <2000 cells/mm 3 95 97 ns <1000 cells/mm 3 84 78 ns Leukopenia <4000 cells/mm 3 97 97 ns <2000 cells/mm 3 76 67 ns Anemia <11 g/dL 88 87 ns <8 g/dL 8 24 <0.001 Infections 18 21 ns Bleeding 6 4 ns Transfusions 25 33 ns Gastrointestinal Nausea and vomiting 94 96 ns Vomiting 82 91 0.007 Other GI side effects 40 48 ns Neurologic Peripheral neuropathies 13 28 0.001 Ototoxicity 12 30 <0.001 Other sensory side effects 4 6 ns Central neurotoxicity 23 29 ns Renal Serum creatinine elevations 7 38 <0.001 Blood urea elevations - - - Hepatic Bilirubin elevations 5 3 ns SGOT elevations 23 16 ns Alkaline phosphatase elevations 29 20 ns Electrolytes loss Sodium - - - Potassium - - - Calcium - - - Magnesium 58 77 <0.001 Other side effects Pain 54 52 ns Asthenia 43 46 ns Cardiovascular 23 30 ns Respiratory 12 11 ns Allergic 10 11 ns Genitourinary 11 13 ns Alopecia ‡ 43 57 0.009 Mucositis 6 11 ns Use as a Single Agent for Secondary Treatment of Advanced Ovarian Cancer
In two prospective, randomized controlled studies in patients with advanced ovarian cancer previously treated with chemotherapy, carboplatin achieved 6 clinical complete responses in 47 patients. The duration of these responses ranged from 45 to 71+ weeks.
-
INDICATIONS
Initial Treatment of Advanced Ovarian Carcinoma
Paraplatin ® injection is indicated for the initial treatment of advanced ovarian carcinoma in established combination with other approved chemotherapeutic agents. One established combination regimen consists of Paraplatin ® and cyclophosphamide. Two randomized controlled studies conducted by the NCIC and SWOG with carboplatin versus cisplatin, both in combination with cyclophosphamide, have demonstrated equivalent overall survival between the two groups (see CLINICAL STUDIES).
There is limited statistical power to demonstrate equivalence in overall pathologic complete response rates and long-term survival (≥ 3 years) because of the small number of patients with these outcomes: the small number of patients with residual tumor < 2 cm after initial surgery also limits the statistical power to demonstrate equivalence in this subgroup.
Secondary Treatment of Advanced Ovarian Carcinoma
Paraplatin ® is indicated for the palliative treatment of patients with ovarian carcinoma recurrent after prior chemotherapy, including patients who have been previously treated with cisplatin.
Within the group of patients previously treated with cisplatin, those who have developed progressive disease while receiving cisplatin therapy may have a decreased response rate.
- CONTRAINDICATIONS
-
WARNINGS
Bone marrow suppression (leukopenia, neutropenia, and thrombocytopenia) is dose-dependent and is also the dose-limiting toxicity. Peripheral blood counts should be frequently monitored during Paraplatin ® treatment and, when appropriate, until recovery is achieved. Median nadir occurs at day 21 in patients receiving single agent carboplatin. In general, single intermittent courses of Paraplatin ® should not be repeated until leukocyte, neutrophil, and platelet counts have recovered.
Since anemia is cumulative, transfusions may be needed during treatment with Paraplatin ®, particularly in patients receiving prolonged therapy.
Bone marrow suppression is increased in patients who have received prior therapy, especially regimens including cisplatin. Marrow suppression is also increased in patients with impaired kidney function. Initial Paraplatin ® dosages in these patients should be appropriately reduced (see DOSAGE AND ADMINISTRATION) and blood counts should be carefully monitored between courses. The use of Paraplatin ® in combination with other bone marrow suppressing therapies must be carefully managed with respect to dosage and timing in order to minimize additive effects.
Carboplatin has limited nephrotoxic potential, but concomitant treatment with aminoglycosides has resulted in increased renal and/or audiologic toxicity, and caution must be exercised when a patient receives both drugs. Clinically significant hearing loss has been reported to occur in pediatric patients when carboplatin was administered at higher than recommended doses in combination with other ototoxic agents.
Paraplatin ® can induce emesis, which can be more severe in patients previously receiving emetogenic therapy. The incidence and intensity of emesis have been reduced by using premedication with antiemetics. Although no conclusive efficacy data exist with the following schedules of Paraplatin ®, lengthening the duration of single intravenous administration to 24 hours or dividing the total dose over 5 consecutive daily pulse doses has resulted in reduced emesis.
Although peripheral neurotoxicity is infrequent, its incidence is increased in patients older than 65 years and in patients previously treated with cisplatin. Pre-existing cisplatin-induced neurotoxicity does not worsen in about 70% of the patients receiving carboplatin as secondary treatment.
Loss of vision, which can be complete for light and colors, has been reported after the use of carboplatin with doses higher than those recommended in the package insert. Vision appears to recover totally or to a significant extent within weeks of stopping these high doses.
As in the case of other platinum-coordination compounds, allergic reactions to carboplatin have been reported. These may occur within minutes of administration and should be managed with appropriate supportive therapy. There is increased risk of allergic reactions including anaphylaxis in patients previously exposed to platinum therapy. (See CONTRAINDICATIONS and ADVERSE REACTIONS: Allergic Reactions.)
High dosages of carboplatin (more than 4 times the recommended dose) have resulted in severe abnormalities of liver function tests.
Paraplatin ® injection may cause fetal harm when administered to a pregnant woman. Carboplatin has been shown to be embryotoxic and teratogenic in rats. There are no adequate and well-controlled studies in pregnant women. If this drug is used during pregnancy, or if the patient becomes pregnant while receiving this drug, the patient should be apprised of the potential hazard to the fetus. Women of childbearing potential should be advised to avoid becoming pregnant.
-
PRECAUTIONS
General
Needles or intravenous administration sets containing aluminum parts that may come in contact with Paraplatin ® injection should not be used for the preparation or administration of the drug. Aluminum can react with carboplatin causing precipitate formation and loss of potency.
Carcinogenesis, Mutagenesis, Impairment of Fertility
The carcinogenic potential of carboplatin has not been studied, but compounds with similar mechanisms of action and mutagenicity profiles have been reported to be carcinogenic. Carboplatin has been shown to be mutagenic both in vitro and in vivo. It has also been shown to be embryotoxic and teratogenic in rats receiving the drug during organogenesis. Secondary malignancies have been reported in association with multi-drug therapy.
Nursing Mothers
It is not known whether carboplatin is excreted in human milk. Because there is a possibility of toxicity in nursing infants secondary to Paraplatin ® treatment of the mother, it is recommended that breast-feeding be discontinued if the mother is treated with Paraplatin ® injection.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established (see WARNINGS: "audiologic toxicity").
Geriatric Use
Of the 789 patients in initial treatment combination therapy studies (NCIC and SWOG), 395 patients were treated with carboplatin in combination with cyclophosphamide. Of these, 141 were over 65 years of age and 22 were 75 years or older. In these trials, age was not a prognostic factor for survival. In terms of safety, elderly patients treated with carboplatin were more likely to develop severe thrombocytopenia than younger patients. In a combined database of 1,942 patients (414 were ≥ 65 years of age) that received single agent carboplatin for different tumor types, a similar incidence of adverse events was seen in patients 65 years and older and in patients less than 65. Other reported clinical experience has not identified differences in responses between elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out. Because renal function is often decreased in the elderly, renal function should be considered in the selection of Paraplatin ® injection dosage (see DOSAGE AND ADMINISTRATION).
-
ADVERSE REACTIONS
For a comparison of toxicities when carboplatin or cisplatin was given in combination with cyclophosphamide, see CLINICAL STUDIES: Use with Cyclophosphamide for Initial Treatment of Ovarian Cancer: Comparative Toxicity.
ADVERSE EXPERIENCES IN PATIENTS WITH OVARIAN CANCER First Line Combination Therapy *
PercentSecond Line Single Agent Therapy †
Percent- * Use with Cyclophosphamide for Initial Treatment of Ovarian Cancer: Data are based on the experience of 393 patients with ovarian cancer (regardless of baseline status) who received initial combination therapy with carboplatin and cyclophosphamide in two randomized controlled studies conducted by SWOG and NCIC (see CLINICAL STUDIES). Combination with cyclophosphamide as well as duration of treatment may be responsible for the differences that can be noted in the adverse experience table.
- † Single Agent Use for the Secondary Treatment of Ovarian Cancer: Data are based on the experience of 553 patients with previously treated ovarian carcinoma (regardless of baseline status) who received single agent carboplatin.
Bone Marrow Thrombocytopenia <100,000/mm 3 66 62 <50,000/mm 3 33 35 Neutropenia <2000 cells/mm 3 96 67 <1000 cells/mm 3 82 21 Leukopenia <4000 cells/mm 3 97 85 <2000 cells/mm 3 71 26 Anemia <11 g/dL 90 90 <8g/dL 14 21 Infections 16 5 Bleeding 8 5 Transfusions 35 44 Gastrointestinal Nausea and vomiting 93 92 Vomiting 83 81 Other GI side effects 46 21 Neurologic Peripheral neuropathies 15 6 Ototoxicity 12 1 Other sensory side effects 5 1 Central neurotoxicity 26 5 Renal Serum creatinine elevations 6 10 Blood urea elevations 17 22 Hepatic Bilirubin elevations 5 5 SGOT elevations 20 19 Alkaline phosphatase elevations 29 37 Electrolytes loss Sodium 10 47 Potassium 16 28 Calcium 16 31 Magnesium 61 43 Other side effects Pain 44 23 Asthenia 41 11 Cardiovascular 19 6 Respiratory 10 6 Allergic 11 2 Genitourinary 10 2 Alopecia 49 2 Mucositis 8 1 In the narrative section that follows, the incidences of adverse events are based on data from 1,893 patients with various types of tumors who received carboplatin as single agent therapy.
Hematologic Toxicity
Bone marrow suppression is the dose-limiting toxicity of Paraplatin ®. Thrombocytopenia with platelet counts below 50,000/mm 3 occurs in 25% of the patients (35% of pretreated ovarian cancer patients); neutropenia with granulocyte counts below 1,000/mm 3 occurs in 16% of the patients (21% of pretreated ovarian cancer patients); leukopenia with WBC counts below 2,000/mm 3 occurs in 15% of the patients (26% of pretreated ovarian cancer patients). The nadir usually occurs about day 21 in patients receiving single agent therapy. By day 28, 90% of patients have platelet counts above 100,000/mm 3; 74% have neutrophil counts above 2,000/mm 3; 67% have leukocyte counts above 4000/mm 3.
Marrow suppression is usually more severe in patients with impaired kidney function. Patients with poor performance status have also experienced a higher incidence of severe leukopenia and thrombocytopenia.
The hematologic effects, although usually reversible, have resulted in infectious or hemorrhagic complications in 5% of the patients treated with carboplatin, with drug related death occurring in less than 1% of the patients. Fever has also been reported in patients with neutropenia.
Anemia with hemoglobin less than 11 g/dL has been observed in 71% of the patients who started therapy with a baseline above that value. The incidence of anemia increases with increasing exposure to Paraplatin ®. Transfusions have been administered to 26% of the patients treated with carboplatin (44% of previously treated ovarian cancer patients).
Bone marrow depression may be more severe when Paraplatin ® is combined with other bone marrow suppressing drugs or with radiotherapy.
Gastrointestinal Toxicity
Vomiting occurs in 65% of the patients (81% of previously treated ovarian cancer patients) and in about one-third of these patients it is severe. Carboplatin, as a single agent or in combination, is significantly less emetogenic than cisplatin; however, patients previously treated with emetogenic agents, especially cisplatin, appear to be more prone to vomiting. Nausea alone occurs in an additional 10% to 15% of patients. Both nausea and vomiting usually cease within 24 hours of treatment and are often responsive to antiemetic measures. Although no conclusive efficacy data exist with the following schedules, prolonged administration of carboplatin, either by continuous 24-hour infusion or by daily pulse doses given for 5 consecutive days, was associated with less severe vomiting than the single-dose intermittent schedule. Emesis was increased when carboplatin was used in combination with other emetogenic compounds. Other gastrointestinal effects observed frequently were pain, in 17% of the patients; diarrhea, in 6%; and constipation, also in 6%.
Neurologic Toxicity
Peripheral neuropathies have been observed in 4% of the patients receiving carboplatin (6% of pretreated ovarian cancer patients) with mild paresthesias occurring most frequently. Carboplatin therapy produces significantly fewer and less severe neurologic side effects than does therapy with cisplatin. However, patients older than 65 years and/or previously treated with cisplatin appear to have an increased risk (10%) for peripheral neuropathies. In 70% of the patients with pre-existing cisplatin-induced peripheral neurotoxicity, there was no worsening of symptoms during therapy with carboplatin. Clinical ototoxicity and other sensory abnormalities such as visual disturbances and change in taste have been reported in only 1% of the patients. Central nervous system symptoms have been reported in 5% of the patients and appear to be most often related to the use of antiemetics.
Although the overall incidence of peripheral neurologic side effects induced by carboplatin is low, prolonged treatment, particularly in cisplatin pretreated patients, may result in cumulative neurotoxicity.
Nephrotoxicity
Development of abnormal renal function test results is uncommon, despite the fact that carboplatin, unlike cisplatin, has usually been administered without high-volume fluid hydration and/or forced diuresis. The incidences of abnormal renal function tests reported are 6% for serum creatinine and 14% for blood urea nitrogen (10% and 22%, respectively, in pretreated ovarian cancer patients). Most of these reported abnormalities have been mild and about one-half of them were reversible.
Creatinine clearance has proven to be the most sensitive measure of kidney function in patients receiving carboplatin, and it appears to be the most useful test for correlating drug clearance and bone marrow suppression. Twenty-seven percent of the patients who had a baseline value of 60 mL/min or more demonstrated a reduction below this value during carboplatin therapy.
Hepatic Toxicity
The incidences of abnormal liver function tests in patients with normal baseline values were reported as follows: total bilirubin, 5%; SGOT, 15%; and alkaline phosphatase, 24%; (5%, 19%, and 37%, respectively, in pretreated ovarian cancer patients). These abnormalities have generally been mild and reversible in about one-half of the cases, although the role of metastatic tumor in the liver may complicate the assessment in many patients. In a limited series of patients receiving very high dosages of carboplatin and autologous bone marrow transplantation, severe abnormalities of liver function tests were reported.
Electrolyte Changes
The incidences of abnormally decreased serum electrolyte values reported were as follows: sodium, 29%; potassium, 20%; calcium, 22%; and magnesium, 29%; (47%, 28%, 31%, and 43%, respectively, in pretreated ovarian cancer patients). Electrolyte supplementation was not routinely administered concomitantly with carboplatin, and these electrolyte abnormalities were rarely associated with symptoms.
Allergic Reactions
Hypersensitivity to carboplatin has been reported in 2% of the patients. These allergic reactions have been similar in nature and severity to those reported with other platinum-containing compounds, ie, rash, urticaria, erythema, pruritus, and rarely bronchospasm and hypotension. Anaphylactic reactions have been reported as part of postmarketing surveillance (see WARNINGS). These reactions have been successfully managed with standard epinephrine, corticosteroid, and antihistamine therapy.
Injection Site Reactions
Injection site reactions, including redness, swelling, and pain, have been reported during postmarketing surveillance. Necrosis associated with extravasation has also been reported.
Other Events
Pain and asthenia were the most frequently reported miscellaneous adverse effects; their relationship to the tumor and to anemia was likely. Alopecia was reported (3%). Cardiovascular, respiratory, genitourinary, and mucosal side effects have occurred in 6% or less of the patients. Cardiovascular events (cardiac failure, embolism, cerebrovascular accidents) were fatal in less than 1% of the patients and did not appear to be related to chemotherapy. Cancer-associated hemolytic uremic syndrome has been reported rarely.
Malaise, anorexia, hypertension, dehydration, and stomatitis have been reported as part of postmarketing surveillance.
- OVERDOSAGE
-
DOSAGE AND ADMINISTRATION
NOTE: Aluminum reacts with carboplatin causing precipitate formation and loss of potency, therefore, needles or intravenous sets containing aluminum parts that may come in contact with the drug must not be used for the preparation or administration of Paraplatin ®.
Single Agent Therapy
Paraplatin ® injection, as a single agent, has been shown to be effective in patients with recurrent ovarian carcinoma at a dosage of 360 mg/m 2 IV on day 1 every 4 weeks (alternatively see Formula Dosing). In general, however, single intermittent courses of carboplatin should not be repeated until the neutrophil count is at least 2,000 and the platelet count is at least 100,000.
Combination Therapy with Cyclophosphamide
In the chemotherapy of advanced ovarian cancer, an effective combination for previously untreated patients consists of:
Paraplatin ®-300 mg/m 2 IV on day 1 every 4 weeks for 6 cycles (alternatively see Formula Dosing).
Cyclophosphamide-600 mg/m 2 IV on day 1 every 4 weeks for 6 cycles. For directions regarding the use and administration of cyclophosphamide please refer to its package insert. (See CLINICAL STUDIES.)
Intermittent courses of Paraplatin ® in combination with cyclophosphamide should not be repeated until the neutrophil count is at least 2,000 and the platelet count is at least 100,000.
Dose Adjustment Recommendations
Pretreatment platelet count and performance status are important prognostic factors for severity of myelosuppression in previously treated patients.
The suggested dose adjustments for single agent or combination therapy shown in the table below are modified from controlled trials in previously treated and untreated patients with ovarian carcinoma. Blood counts were done weekly, and the recommendations are based on the lowest post-treatment platelet or neutrophil value.
Platelets Neutrophils Adjusted Dose *
(From Prior Course)- * Percentages apply to Paraplatin ® injection as a single agent or to both Paraplatin ® and cyclophosphamide in combination. In the controlled studies, dosages were also adjusted at a lower level (50% to 60%) for severe myelosuppression. Escalations above 125% were not recommended for these studies.
> 100,000 > 2,000 125% 50-100,000 500-2,000 No Adjustment < 50,000 < 500 75% Paraplatin ® injection is usually administered by an infusion lasting 15 minutes or longer. No pre- or post-treatment hydration or forced diuresis is required.
Patients with Impaired Kidney Function
Patients with creatinine clearance values below 60 mL/min are at increased risk of severe bone marrow suppression. In renally-impaired patients who received single agent carboplatin therapy, the incidence of severe leukopenia, neutropenia, or thrombocytopenia has been about 25% when the dosage modifications in the table below have been used.
Baseline Creatinine Clearance Recommended Dose on Day 1 41 - 59 mL/min 250 mg/m 2 16 - 40 mL/min 200 mg/m 2 The data available for patients with severely impaired kidney function (creatinine clearance below 15 mL/min) are too limited to permit a recommendation for treatment.
These dosing recommendations apply to the initial course of treatment. Subsequent dosages should be adjusted according to the patient's tolerance based on the degree of bone marrow suppression.
Formula Dosing
Another approach for determining the initial dose of Paraplatin ® injection is the use of mathematical formulae, which are based on a patient's pre-existing renal function or renal function and desired platelet nadir. Renal excretion is the major route of elimination for carboplatin. (See CLINICAL PHARMACOLOGY.) The use of dosing formulae, as compared to empirical dose calculation based on body surface area, allows compensation for patient variations in pretreatment renal function that might otherwise result in either underdosing (in patients with above average renal function) or overdosing (in patients with impaired renal function).
A simple formula for calculating dosage, based upon a patient's glomerular filtration rate (GFR in mL/min) and Paraplatin ® injection target area under the concentration versus time curve (AUC in mg/mLmin), has been proposed by Calvert. In these studies, GFR was measured by 51Cr-EDTA clearance.
CALVERT FORMULA FOR CARBOPLATIN DOSING
Total Dose (mg) = (target AUC) × (GFR + 25)
Note: With the Calvert formula, the total dose of Paraplatin ® is calculated in mg, not mg/m 2.
The target AUC of 4 mg/mLmin to 6 mg/mLmin using single-agent carboplatin appears to provide the most appropriate dose range in previously treated patients. This study also showed a trend between the AUC of single-agent carboplatin administered to previously treated patients and the likelihood of developing toxicity.
% Actual Toxicity in Previously Treated Patients AUC (mg/mLmin) Gr 3 or Gr 4 Thrombocytopenia Gr 3 or Gr 4 Leukopenia 4 to 5 16% 13% 6 to 7 33% 34% Geriatric Dosing
Because renal function is often decreased in elderly patients, formula dosing of Paraplatin ® based on estimates of GFR should be used in elderly patients to provide predictable plasma Paraplatin ® AUCs and thereby minimize the risk of toxicity.
PREPARATION OF INTRAVENOUS SOLUTIONS
Paraplatin ® injection is a premixed aqueous solution of 10 mg/mL carboplatin.
Paraplatin ® aqueous solution can be further diluted to concentrations as low as 0.5 mg/mL with 5% Dextrose in Water (D 5W) or 0.9% Sodium Chloride Injection, USP.
When prepared as directed, Paraplatin ® aqueous solutions are stable for 8 hours at room temperature (25°C). Since no antibacterial preservative is contained in the formulation, it is recommended that Paraplatin ® aqueous solutions be discarded 8 hours after dilution.
-
HOW SUPPLIED
Each mL of Paraplatin ® contains 10 mg of carboplatin, USP in water for injection and is available in individual cartons as follows:
NDC Number Contents Size 69448-005-31 50 mg 5 mL Multidose Vial 69448-005-33 150 mg 15 mL Multidose Vial 69448-005-34 450 mg 45 mL Multidose Vial 69448-005-12 600 mg 60 mL Multidose Vial STORAGE
Unopened vials of Paraplatin ® injection are stable to the date indicated on the package when stored at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
PROTECT FROM LIGHT.
Paraplatin ® (carboplatin) injection multidose vials maintain microbial, chemical, and physical stability for up to 14 days at 25°C following multiple needle entries.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration. Solutions for infusion should be discarded 8 hours after preparation.
HANDLING AND DISPOSAL
Caution should be exercised in handling and preparing Paraplatin ® (carboplatin) injection. Several guidelines on this subject have been published. 1-4
To minimize the risk of dermal exposure, always wear impervious gloves when handling vials containing Paraplatin ® (carboplatin) injection. If Paraplatin ® (carboplatin) injection contacts the skin, immediately wash the skin thoroughly with soap and water. If Paraplatin ® (carboplatin) injection contacts mucous membranes, the membranes should be flushed immediately and thoroughly with water. More information is available in the references listed below.
-
REFERENCES
- NIOSH Alert: Preventing occupational exposures to antineoplastic and other hazardous drugs in healthcare settings. 2004. U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, DHHS (NIOSH) Publication No. 2004-165.
- OSHA Technical Manual, TED 1-0.15A, Section VI: Chapter 2. Controlling occupational exposure to hazardous drugs. OSHA, 1999. http://www.osha.gov/dts/osta/otm/otm_vi/otm_vi_2.html
- American Society of Health-System Pharmacists. ASHP guidelines on handling hazardous drugs. Am J Health-Syst Pharm. 2006;63:1172-1193.
- Polovich M, White JM, Kelleher LO, eds. 2005. Chemotherapy and biotherapy guidelines and recommendations for practice. 2nd ed. Pittsburgh, PA: Oncology Nursing Society.
Manufactured for:
Accord BioPharma Inc.,
1009, Slater Road,
Suite 210-B,
Durham, NC 27703,
USA.Manufacutered by:
Intas Pharmaceuticals Limited,
Plot No. 5 to14, Pharmez,
Sarkhej-Bavla, National Highway No. 8-A
Near Village Matoda, Tal Sanand,
Ahmedabad - 382 213, Gujarat, India.Issued: August 2019
-
Patient Information
Paraplatin ® (par ah pla tin) Injection
Rx only
Read this entire leaflet carefully. Keep it for future reference.
This information will help you learn more about Paraplatin ®. It cannot, however, cover all the possible warnings or side effects relating to Paraplatin ®, and it does not list all of the benefits and risks of Paraplatin ®. Your doctor should always be your first choice for detailed information about your medical condition and your treatment. Be sure to ask your doctor about any questions you may have.
What is cancer?
Under normal conditions, the cells in your body divide and grow in an orderly, controlled fashion. Cell division and growth are necessary for the human body to perform its functions and to repair itself. Cancer cells are different from normal cells because they are not able to control their growth. The reasons for this abnormal growth are not yet fully understood.
A tumor is a mass of unhealthy cells that are dividing and growing fast and in an uncontrolled way. When a tumor invades surrounding healthy body tissue it is known as a malignant tumor. A malignant tumor can spread (metastasize) from its original location to other parts of the body.
What is Paraplatin ®?
Paraplatin ® is a medicine that is used to treat cancer of the ovaries. It acts by interfering with the division of rapidly multiplying cells, particularly cancer cells.
Who should not take Paraplatin ®?
Treatment with Paraplatin ® is not recommended if you:
- are allergic to Paraplatin ® or other platinum-containing products;
- have a weakened blood-forming system (bone marrow depression) or significant bleeding;
- are pregnant, intend to become pregnant, or are breastfeeding a baby.
How is Paraplatin ® used?
Only a professional experienced in the use of cancer drugs should give you this medication. Paraplatin ® is given by dripping the medicine slowly and directly into a vein (intravenous infusion) for 15 minutes or longer. Your doctor will determine the dose of Paraplatin ® for you based on your weight, height, and kidney function. Paraplatin ® may be given alone or with other drugs. Treatment is usually repeated every four weeks for a number of cycles.
Before and after Paraplatin ® treatment, your doctor may give you medication to lessen the nausea and vomiting associated with this cancer treatment
What should you tell your doctor before starting treatment with Paraplatin ®?
Discuss the benefits and risks of Paraplatin ® with your doctor before beginning treatment.
Be sure to inform your doctor:
- If you are allergic to Paraplatin ® or other platinum-containing products;
- If you are or intend to become pregnant, since Paraplatin ® may harm the developing fetus. It is important to use effective birth control while you are being treated with Paraplatin ®.
- If you are breastfeeding, since nursing infants may be exposed to Paraplatin ® in this way;
- If you are taking other medicines, including all prescription and non-prescription (over-the-counter) drugs, since Paraplatin ® may affect the action of other medicines;
- If you have any other medical problems, especially chicken pox (including recent exposure to adults or children with chicken pox), shingles, hearing problems, infection, or kidney disease, since treatment with Paraplatin ® increases the risk and severity of these conditions.
What should I avoid while taking Paraplatin ®?
If you are pregnant or think you might be pregnant, or if you are breastfeeding, let your doctor know right away. Paraplatin ® may harm your developing fetus or breastfeeding baby. If you are a woman of childbearing age, you should use birth control to avoid getting pregnant while you are taking Paraplatin ®.
You should avoid contact with adults and children who have infections, and tell your doctor right away if you show signs of infection such as cough, fever, and/or chills. Also, while you are being treated with Paraplatin ® or after you stop treatment, first check with your doctor before getting any immunizations (vaccinations). Avoid contact with adults or children who have received oral polio vaccine since they can pass the polio virus to you.
What are the possible side effects of Paraplatin ®?
Paraplatin ® may cause unwanted effects, particularly because Paraplatin ® interferes with the growth of normal cells as well as cancer cells. For example, the occurrence of another cancer (secondary malignancy) has been reported in patients receiving cancer chemotherapy with multiple drugs. It is not always possible to tell whether such effects are caused by Paraplatin ®, another drug you may be taking, or your illness. Because some of these effects may be serious, you will need close medical supervision during treatment withParaplatin ®.
The most serious side effects of Paraplatin ® are:
- bleeding and reduced blood cells, including reduced red blood cells (anemia) and platelets (needed for proper blood clotting), which may be severe enough to require blood transfusion. You should tell your doctor right away if you notice any unusual bruising or bleeding, including black tarry stools or blood in the urine.
- infection – Paraplatin ® can temporarily lower the number of white blood cells in your blood, increasing the risk of infection;
- life-threatening allergic reaction – during and after treatment the doctor or nurse will observe you carefully for signs of allergic reaction;
- kidney and liver problems;
- loss of hearing or ringing in the ears;
Contact your doctor right away if you experience any of these effects, or notice effects that worry you or are troublesome.
Of the less serious side effects associated with Paraplatin ® treatment, the most common are nausea, vomiting, diarrhea, loss of appetite, hair loss and numbness, tingling, burning, or pain in the hands and feet.
This medicine was prescribed for your particular condition. It must be given under close medical supervision by a doctor trained in the use of drugs for the treatment of cancer.
This summary does not include everything there is to know about Paraplatin ®. Medicines are sometimes prescribed for purposes other than those listed in patient leaflets. If you have questions or concerns, or want more information about Paraplatin ®, your physician and pharmacist have the complete prescribing information upon which this information is based. You may want to read it and discuss it with your doctor. Remember, no written summary can replace careful discussion with your doctor.
Manufactured for:
Accord BioPharma Inc.,
1009, Slater Road,
Suite 210-B,
Durham, NC 27703,
USA.Manufacutered by:
Intas Pharmaceuticals Limited,
Plot No. 5 to14, Pharmez,
Sarkhej-Bavla, National Highway No. 8-A
Near Village Matoda, Tal Sanand,
Ahmedabad - 382 213, Gujarat, India.51 3873 1 722455
Issued: August 2019
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
NDC: 69448-005-31 Rx only
PARAPLATIN ® (carboplatin) Injection 50 mg/5 mL Vial – Container Label
50 mg/5 mL
(10 mg/mL)For Intravenous Use
5 mL Multiple Dose Vial
CAUTION: Cytotoxic Agent
NDC: 69448-005-31 Rx only
PARAPLATIN ® (carboplatin) Injection 50 mg/5 mL Vial – Carton Label
50 mg/5 mL
(10 mg/mL)For Intravenous Use
5 mL Multiple Dose Vial
CAUTION: Cytotoxic AgentPHARMACIST: PLEASE DISPENSE WITH ATTACHED PATIENT INFORMATION LEAFLET.

NDC: 69448-005-33 Rx only
PARAPLATIN ® (carboplatin) Injection 150 mg/15 mL Vial – Container Label
150 mg/15 mL
(10 mg/mL)For Intravenous Use
15 mL Multiple Dose Vial
CAUTION: Cytotoxic Agent
NDC: 69448-005-33 Rx only
PARAPLATIN ® (carboplatin) Injection 150 mg/15 mL Vial – Carton Label
150 mg/15 mL
(10 mg/mL)For Intravenous Use
15 mL Multiple Dose Vial
CAUTION: Cytotoxic AgentPHARMACIST: PLEASE DISPENSE WITH ATTACHED PATIENT INFORMATION LEAFLET.
NDC: 69448-005-34 Rx only
PARAPLATIN ® (carboplatin) Injection 450 mg/45 mL Vial – Container Label
450 mg/45 mL
(10 mg/mL)For Intravenous Use
45 mL Multiple Dose Vial
CAUTION: Cytotoxic Agent
NDC: 69448-005-34 Rx only
PARAPLATIN ® (carboplatin) Injection 450 mg/45 mL Vial – Carton Label
450 mg/45 mL
(10 mg/mL)For Intravenous Use
45 mL Multiple Dose Vial
CAUTION: Cytotoxic AgentPHARMACIST: PLEASE DISPENSE WITH ATTACHED PATIENT INFORMATION LEAFLET.
NDC: 69448-005-12 Rx only
PARAPLATIN ® (carboplatin) Injection 600 mg/60 mL Vial – Container Label
600 mg/60 mL
(10 mg/mL)For Intravenous Use
60 mL Multiple Dose Vial
CAUTION: Cytotoxic Agent
NDC: 69448-005-12 Rx only
PARAPLATIN ® (carboplatin) Injection 600 mg/60 mL Vial – Carton Label
600 mg/60 mL
(10 mg/mL)For Intravenous Use
60 mL Multiple Dose Vial
CAUTION: Cytotoxic AgentPHARMACIST: PLEASE DISPENSE WITH ATTACHED PATIENT INFORMATION LEAFLET.

-
INGREDIENTS AND APPEARANCE
PARAPLATIN
carboplatin injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 69448-005 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CARBOPLATIN (UNII: BG3F62OND5) (CARBOPLATIN - UNII:BG3F62OND5) CARBOPLATIN 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69448-005-31 1 in 1 CARTON 02/12/2020 1 5 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product 2 NDC: 69448-005-33 1 in 1 CARTON 02/12/2020 2 15 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product 3 NDC: 69448-005-34 1 in 1 CARTON 02/12/2020 3 45 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product 4 NDC: 69448-005-12 1 in 1 CARTON 02/12/2020 4 60 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA206775 02/12/2020 Labeler - Accord BioPharma Inc. (079636487) Registrant - Accord Healthcare Inc. (604222237) Establishment Name Address ID/FEI Business Operations Intas Pharmaceuticals Limited 915837971 manufacture(69448-005) , analysis(69448-005)
Trademark Results [PARAPLATIN]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 PARAPLATIN 88057968 not registered Live/Pending |
Accord Healthcare, Inc. 2018-07-30 |
 PARAPLATIN 73552929 1384946 Dead/Cancelled |
BRISTOL-MYERS COMPANY 1985-08-12 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.