DETECTNET- copper cu 64 dotatate injection, solution
Detectnet by
Drug Labeling and Warnings
Detectnet by is a Prescription medication manufactured, distributed, or labeled by CURIUM US LLC, Curium US LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use DETECTNET TMsafely and effectively. See full prescribing information for DETECTNET.
DETECTNET (copper Cu 64 dotatate injection), for intravenous use
Initial U.S. Approval: 2020INDICATIONS AND USAGE
Detectnet is a radioactive diagnostic agent indicated for use with positron emission tomography (PET) for localization of somatostatin receptor positive neuroendocrine tumors (NETs) in adult patients. ( 1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
- Injection: 148 MBq (4 mCi) (37 MBq (1 mCi) per 1 mL) of copper Cu 64 dotatate in a single-dose vial.( 3)
CONTRAINDICATIONS
None ( 4).
WARNINGS AND PRECAUTIONS
- Radiation Risk: Ensure safe handling and preparation procedures to protect patients and health care workers from unintentional radiation exposure ( 5.1). Advise patients to hydrate before and after administration and to void frequently after administration. ( 2.3)
- Hypersensitivity Reactions: Most reported reactions were rash and pruritus and reversible either spontaneously or with routine symptomatic management. ( 5.2)
- Risk for Image Misinterpretation: Uptake of Detectnet can be seen in a variety of tumor types other than NETs, in other pathologic conditions, and as a normal physiologic variant (e.g., uncinate process of the pancreas). ( 5.3)
ADVERSE REACTIONS
Reported adverse reactions include nausea, vomiting, and flushing. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact CURIUM US LLC at 1-866-789-2211 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Somatostatin Analogs: Somatostatin analogs competitively bind to the same somatostatin receptors as copper Cu 64 dotatate and may affect imaging. Image patients just prior to dosing with somatostatin analogs. For patients on long-acting somatostatin analogs, a wash-out period of 28 days is recommended prior to imaging. For patients on short-acting somatostatin analogs, a washout period of 2 days is recommended prior to imaging. ( 2.3, 7.1)
USE IN SPECIFIC POPULATIONS
Lactation: Advise patients to interrupt breastfeeding for 12 hours after Detectnet administration. ( 8.2)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 1/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Radiation Safety – Drug Handling
2.2 Recommended Dosage and Administration Instructions
2.3 Patient Preparation
2.4 Image Acquisition
2.5 Image Interpretation
2.6 Radiation Dosimetry
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Radiation Risk
5.2 Hypersensitivity Reactions
5.3 Risk for Image Misinterpretation
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Somatostatin Analogs
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
11.1 Chemical Characteristics
11.2 Physical Characteristics
11.3 External Radiation
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Radiation Safety – Drug Handling
Handle Detectnet with appropriate safety measures to minimize radiation exposure [see Warnings and Precautions ( 5.1)] . Use waterproof gloves, effective radiation shielding and appropriate safety measures when preparing and handling Detectnet.
Radiopharmaceuticals should be used by or under the control of physicians who are qualified by specific training and experience in the safe use and handling of radionuclides, and whose experience and training have been approved by the appropriate governmental agency authorized to license the use of radionuclides.
2.2 Recommended Dosage and Administration Instructions
Recommended Dosage
In adults, the recommended amount of radioactivity to be administered for PET imaging is 148 MBq (4 mCi) administered as an intravenous injection over a period of approximately 1 minute.
Administration
- Use Detectnet within 2 hours after calibration time.
- Use aseptic technique and radiation shielding when withdrawing and administering Detectnet.
- Inspect Detectnet visually for particulate matter and discoloration before administration. Do not use the drug if the solution contains particulate matter or is discolored.
- Calculate the necessary volume to administer based on measured activity, volume, calibration time, and date.
- Use a dose calibrator to measure the patient dose immediately prior to administration of Detectnet.
- After injection of Detectnet, administer an intravenous flush of 0.9% sodium chloride injection, USP.
- Dispose of any unused drug in a safe manner in compliance with applicable regulations.
2.3 Patient Preparation
Somatostatin Analogs
Image patients just prior to dosing with somatostatin analogs.
For patients on long-acting somatostatin analogs, a wash-out period of 28 days is recommended prior to imaging.
For patients on short-acting somatostatin analogs, a washout period of 2 days is recommended prior to imaging [see Drug Interactions ( 7.1)] .
Patient Hydration
Instruct patients to drink water to ensure adequate hydration prior to administration of Detectnet and to continue to drink and void frequently during the first hours following administration to reduce radiation exposure [see Warnings and Precautions ( 5.1)] .
Pregnancy Status
Assessment of pregnancy status is recommended in females of reproductive potential before administering Detectnet.
2.4 Image Acquisition
For Detectnet PET imaging, a whole-body acquisition from the skull vertex to mid-thigh is recommended. Image acquisition can begin between 45 to 90 minutes after the intravenous administration of Detectnet. Adapt Detectnet uptake time and scan duration according to the equipment used and the patient and tumor characteristics, to obtain the optimal image quality.
2.5 Image Interpretation
Copper Cu 64 dotatate binds to somatostatin receptors. Based upon the intensity of the signals, PET images obtained using copper Cu 64 dotatate injection indicate the presence and density of somatostatin receptors in tissues. Uptake can also be seen in a variety of non-NET tumors that contain somatostatin receptors or as a normal physiologic variant [see Warnings and Precautions ( 5.3)] . NET tumors that do not bear somatostatin receptors will not be visualized.
2.6 Radiation Dosimetry
Estimated radiation absorbed doses per injected activity for organs and tissues of adult patients following an intravenous administration of copper Cu 64 dotatate injection are shown in Table 1.
Table 1. Estimated radiation absorbed dose per injected activity in selected organs with copper Cu 64 dotatate injection - * Mean of 5 patients.
Target Organ Mean *absorbed dose (mGy/MBq) Adrenals 0.137 Brain 0.013 Breasts 0.013 Gallbladder wall 0.040 Lower large intestine wall 0.043 Small intestine 0.066 Stomach wall 0.019 Upper large intestine wall 0.022 Heart wall 0.019 Kidneys 0.139 Liver 0.161 Lungs 0.017 Muscle 0.019 Ovaries 0.019 Pancreas 0.093 Red marrow 0.027 Osteogenic cells 0.034 Skin 0.012 Spleen 0.115 Testes 0.014 Thymus 0.015 Thyroid 0.014 Urinary bladder wall 0.037 Uterus 0.019 Total body 0.025 Effective dose (mSv/MBq) 0.032 The effective radiation dose resulting from the administration of 148 MBq (4 mCi) to an adult is about 4.7 mSv. For an administered activity of 148 MBq (4 mCi) the typical radiation dose to the critical organs, which are the liver, the kidneys/adrenals, and the spleen, are about 24 mGy, 21 mGy and 17 mGy, respectively. Because the spleen has one of the highest physiological uptakes, higher uptake and radiation dose to other organs or pathologic tissues may occur in patients with splenectomy.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Radiation Risk
Diagnostic radiopharmaceuticals, including Detectnet, contribute to a patient’s overall long-term cumulative radiation exposure. Long-term cumulative radiation exposure is associated with an increased risk of cancer. Ensure safe handling and preparation procedures to protect patients and health care workers from unintentional radiation exposure. Advise patients to hydrate before and after administration and to void frequently after administration [see Dosage and Administration ( 2.1, 2.3)] .
5.2 Hypersensitivity Reactions
Hypersensitivity reactions following administration of somatostatin receptor imaging agents predominantly consisted of cutaneous reactions such as rash and pruritus. Reactions reversed either spontaneously or with routine symptomatic management. Less frequently hypersensitivity reactions included angioedema or cases with features of anaphylaxis.
5.3 Risk for Image Misinterpretation
The uptake of copper Cu 64 dotatate reflects the level of somatostatin receptor density in NETs, however, uptake can also be seen in a variety of other tumors that also express somatostatin receptors. Increased uptake might also be seen in other non-cancerous pathologic conditions that express somatostatin receptors including thyroid disease or in subacute inflammation, or might occur as a normal physiologic variant (e.g. uncinate process of the pancreas) [see Dosage and Administration ( 2.5)] .
A negative scan after the administration of Detectnet in patients who do not have a history of NET disease does not rule out disease [see Clinical Studies ( 14)] .
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Hypersensitivity reactions [see Warnings and Precautions ( 5.2)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In safety and efficacy trials, 71 subjects received a single dose of Detectnet. Of these 71 subjects, 21 were healthy volunteers and the remainder were patients with known or suspected NET.
The following adverse reactions occurred at a rate of < 2%:
- Gastrointestinal Disorders:nausea, vomiting
- Vascular Disorders:flushing
In published clinical experience, 126 patients with known history of NET received a single dose of copper Cu 64 dotatate injection. Four patients were reported to have experienced nausea immediately after injection.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of other somatostatin receptor imaging agents. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to the drug.
Immune System Disorders:Hypersensitivity reactions, predominantly rash, pruritus, less frequently angioedema or features of anaphylaxis
-
7 DRUG INTERACTIONS
7.1 Somatostatin Analogs
Non-radioactive somatostatin analogs and copper Cu 64 dotatate competitively bind to somatostatin receptors (SSTR2). Image patients just prior to dosing with somatostatin analogs. For patients on long-acting somatostatin analogs, a wash-out period of 28 days is recommended prior to imaging. For patients on short-acting somatostatin analogs, a washout period of 2 days is recommended prior to imaging [see Dosage and Administration ( 2.3)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
All radiopharmaceuticals, including Detectnet have the potential to cause fetal harm depending on the fetal stage of development and the magnitude of the radiation dose. Advise a pregnant woman of the potential risks of fetal exposure to radiation from administration of Detectnet.
There are no data on Detectnet use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. No animal reproduction studies have been conducted with copper Cu 64 dotatate injection.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defects, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
8.2 Lactation
Risk Summary
There are no data on the presence of copper Cu 64 dotatate in human milk, the effect on the breastfed infant, or the effect on milk production. Lactation studies have not been conducted in animals.
Advise a lactating woman to interrupt breastfeeding for 12 hours after Detectnet administration in order to minimize radiation exposure to a breastfed infant.
8.4 Pediatric Use
The safety and effectiveness of Detectnet have not been established in pediatric patients.
8.5 Geriatric Use
Clinical studies of Detectnet did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
-
10 OVERDOSAGE
In the event of a radiation overdose, the absorbed dose to the patient should be reduced where possible by increasing the elimination of the radionuclide from the body by reinforced hydration and frequent bladder voiding. A diuretic might also be considered. If possible, estimation of the radioactive dose given to the patient should be performed.
-
11 DESCRIPTION
11.1 Chemical Characteristics
Detectnet contains copper Cu 64 dotatate, which is a radioactive diagnostic drug for use with PET imaging. Chemically, copper Cu 64 dotatate is described as copper (Cu 64)-N-[(4,7,10-Tricarboxymethyl-1,4,7,10- tetraazacyclododec-1-yl) acetyl]-Dphenylalanyl-L-cysteinyl-L-tyrosyl-D-tryptophanyl-L-lysyl-L- threoninyl-L-cysteinyl-L-threonine-cyclic (2-7) disulfide. The molecular weight is 1497.2 Daltons and the following is the structural formula:
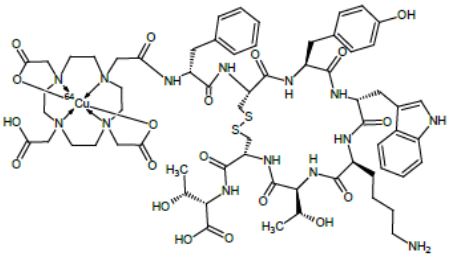
Detectnet is a sterile, clear, colorless to yellow solution for intravenous use. Each 10 mL single-dose vial contains 148 MBq (4 mCi) of copper Cu 64 dotatate at calibration date and time in 4 mL solution volume. Additionally, each mL of the solution contains 40 mg ascorbic acid, 0.05 ml of dehydrated alcohol, USP (ethanol) in sterile water for injection, USP. The pH is adjusted with sodium hydroxide, hydrochloric acid and is between 5.5 to 7.5.
11.2 Physical Characteristics
Table 2 and Table 3 display the principal radiation emission data and physical decay of copper Cu 64.
Copper Cu 64 decays with a half-life t 1/2=12.7 hours:
- 17.6% by positron emission to Ni 64 followed by emission of two 511 keV annihilation photons,
- 38.5% by beta decay to Zn 64,
- 43.8% by electron capture to Ni 64, and
- 0.475% by gamma radiation/internal conversion.
Table 2. Principal radiation emission data (>1%) of copper Cu 64 Radiation/Emission % Disintegration Mean Energy (keV) Positron (β +) 17.6 278 Beta (β -) 38.5 190.7 Gamma (γ) 35.7
0.48511
1346Table 3. Physical decay chart of copper Cu 64 Hours Fraction Remaining Hours Fraction Remaining 0 1.00 18 0.374 1 0.947 24 (1 day) 0.270 3 0.849 36 (1.5 days) 0.140 6 0.721 48 (2 days) 0.073 9 0.612 72 (3 days) 0.020 12 0.520 96 (4 days) 0.005 11.3 External Radiation
Gamma constant: 3.6 X 10 -5mSv/hr per MBq at 1 meter (0.133 mrem/hr per mCi at 1 meter)
Table 4 displays the radiation attenuation by lead shielding of copper Cu 64.Table 4. Radiation attenuation of copper Cu 64 by lead shielding Shield Thickness cm of
Lead (Pb)Coefficient of
Attenuation0.51 0.5 1.60 0.1 3.45 0.01 6.83 0.001 -
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Copper Cu 64 dotatate binds to somatostatin receptors with highest affinity for subtype 2 receptors (SSTR2). It binds to cells that express somatostatin receptors including malignant neuroendocrine cells, which overexpress SSTR2 receptors. Copper Cu 64 is a positron (β +) emitting radionuclide with an emission yield that allows positron emission tomography (PET) imaging.
12.2 Pharmacodynamics
The relationship between copper Cu 64 dotatate plasma concentrations and successful imaging was not explored in clinical trials.
12.3 Pharmacokinetics
Distribution
After 1 to 3 hours of a single dose administration of copper Cu 64 dotatate injection, the maximum radioactivity is observed in adrenal glands, kidney, pituitary glands, spleen, and liver.
Elimination
Metabolism
The metabolism of copper Cu 64 dotatate is unknown.
Excretion
Following a single intravenous dose (4.15 ± 0.13 mCi) of Detectnet (n = 6), between 16% to 40% radioactivity of the injected dose was recovered in urine over a 6-hour collection time.
Specific Populations
The effect of hepatic impairment or renal impairment on copper Cu 64 dotatate pharmacokinetics has not been studied.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity and mutagenicity studies have not been conducted with copper Cu 64 dotatate injection; however, radiation is a carcinogen and mutagen.
No animal studies were conducted to determine the effects of copper Cu 64 dotatate on fertility or embryology.
-
14 CLINICAL STUDIES
The efficacy of Detectnet was established in two single-center, open-label studies. Study 1 prospectively evaluated a total of 63 subjects, including 42 patients with known or suspected NET based on histology, conventional imaging, or clinical evaluations and 21 healthy volunteers. Of the 42 patients, 37 (88%) had a history of NETs at the time of Detectnet imaging. Among the total study population of 63 subjects, 28 (44%) were men and 35 (56%) were women with most subjects being white (86%). The mean age of the subjects was 54 years (range 25 to 82 years).
Detectnet images from each subject were interpreted as either positive or negative for NET by three independent readers who were blinded to clinical information and other imaging results. PET imaging results were compared to a composite reference standard consisting of a single oncologist’s blinded assessment of subject diagnosis based on available histopathology results, reports of conventional imaging (MRI, contrast CT, bone scintigraphy, F 18 fludeoxyglucose PET/CT, F 18 sodium fluoride PET/CT, In 111 pentetreotide SPECT/CT, Ga 68 dotatate PET/CT) performed within 8 weeks prior to Detectnet imaging, and clinical and laboratory data including chromogranin A and serotonin levels. The proportion of subjects positive for disease per composite reference who were identified as positive by Detectnet imaging was used to quantify positive percent agreement. The proportion of subjects without disease per composite reference who were identified as negative by Detectnet imaging was used to quantify negative percent agreement. Table 5 shows the performance of Detectnet in the detection of NET for Study 1.
Table 5: Performance of Detectnet in the detection of NET by reader in Study 1 n: number of patients, CI: confidence interval, *Reader 1 interpreted one of the 63 PET scans as “not evaluable”, **Wilson score interval with continuity correction
NET status as identified by reader
Reference
Positive
Negative
Reader 1
(n=62)*
Positive
30
1
Negative
3
28
Percent Reader Agreement
(95% CI)**
91 (75, 98)
97 (80, 99)
Reader 2
(n=63)
Positive
30
6
Negative
3
24
Percent Reader Agreement
(95% CI)**
91 (75, 98)
80 (61, 92)
Reader 3
(n=63)
Positive
30
3
Negative
3
27
Percent Reader Agreement
(95% CI)**
91 (75, 98)
90 (72, 97)
Study 2 showed similar performance through retrospective analysis of published data collected in 112 patients (63 males, 49 females; mean age 62 years, range 30 to 84 years) with a known history of NET.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
Detectnet (NDC: 69945-064-01) is supplied as a sterile, clear, colorless to yellow solution in a 10 mL single-dose vial containing 148 MBq (4 mCi) (37 MBq (1 mCi) per mL) of copper Cu 64 dotatate at calibration date and time.
The sealed vial is contained in a shielded (lead) container for radiation protection. The product is shipped in a Type A package.
Discard unused portion from the single-patient use vial.
Storage and Handling
Store Detectnet in an upright position within the lead shielding to protect handlers from exposure to radiation.
Store Detectnet at controlled room temperature 20 °C to 25 °C (68 °F to 77 °F). Do not use and discard Detectnet 2 hours after the calibration date and time.
This radiopharmaceutical is for distribution and use by persons under license by the U.S. Nuclear Regulatory Commission or the relevant regulatory authority of an Agreement State. Store and dispose of Detectnet in compliance with the appropriate regulations of the government agency authorized to license the use of this radionuclide.
-
17 PATIENT COUNSELING INFORMATION
Radiation Risk
Advise patients to drink water to ensure adequate hydration prior to their PET study and recommend they drink and urinate as often as possible during the first hours following the administration of Detectnet, in order to reduce radiation exposure [see Dosage and Administration ( 2.3) and Warnings and Precautions ( 5.1)] .
Pregnancy
Advise a pregnant woman of the potential risks of fetal exposure to radiation doses with Detectnet [see Use in Specific Populations ( 8.1)] .
Lactation
Advise a lactating woman to interrupt breastfeeding for 12 hours after Detectnet administration in order to minimize radiation exposure to a breastfed infant [see Use in Specific Populations ( 8.2)] .
Manufactured, Packed and Distributed by:
Curium US LLC
2703 Wagner Place
Maryland Heights, MO 63043© 2025 Curium US LLC
Detectnet TM, Curium TM, and the Curium logo are trademarks of a Curium company. -
Principal Display Panel - Vial
Detect netTM Sterile
(copper Cu 64 dotatate injection) NDC: 69945-064-01148 MBq (4 mCi) per 4 mL at calibration
(37 MBq (1 mCi) per 1 mL)
For Intravenous Use Only
Single-Dose Vial - Discard Unused Portion.Cal. time:1700 CT Exp. time:1900 CT
Cal. date: Exp. date:
Lot #:
-
Principal Display Panel - Can
Detect netTM
(copper Cu 64 dotatate injection) NDC: 69945-064-01148 MBq (4 mCi) per 4 mL at calibration
(37 MBq (1 mCi) per 1 mL)For Intravenous Use Only.
Single-Dose Vial - Discard Unused Portion.Rx only
Recommended adult dosage: 148 MBq (4 mCi) - See Prescribing
Information.
Each mL contains: 37 MBq (1 mCi) of copper Cu 64 dotatate at
calibration date and time, 40 mg ascorbic acid, 0.05 ml of dehydrated
alcohol (ethanol) in sterile water for injection. Sodium hydroxide
and / or hydrochloric acid is added for pH adjustment.Store upright at controlled room temperature 20°C to 25°C (68°F to 77°F)
in a shielded container.CURIUM TM
Manufactured, packed, and distributed by:
Curium US LLC. 2703 Wagner Place, Maryland Heights, MO 63043
-
INGREDIENTS AND APPEARANCE
DETECTNET
copper cu 64 dotatate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 69945-064 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength COPPER OXODOTREOTIDE CU-64 (UNII: N3858377KC) (COPPER OXODOTREOTIDE CU-64 - UNII:N3858377KC) COPPER OXODOTREOTIDE CU-64 1 mCi in 1 mL Inactive Ingredients Ingredient Name Strength ASCORBIC ACID (UNII: PQ6CK8PD0R) 40 mg in 1 mL ALCOHOL (UNII: 3K9958V90M) 0.05 mL in 1 mL WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69945-064-01 1 in 1 CAN 09/14/2020 1 4 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA213227 09/14/2020 Labeler - CURIUM US LLC (079875617) Establishment Name Address ID/FEI Business Operations Curium US LLC 557570652 manufacture(69945-064) , api manufacture(69945-064) , analysis(69945-064)
Trademark Results [Detectnet]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 DETECTNET 88444213 not registered Live/Pending |
CURIUM US LLC 2019-05-23 |
 DETECTNET 78002386 not registered Dead/Abandoned |
Larkin, Jane M. 2000-04-03 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.