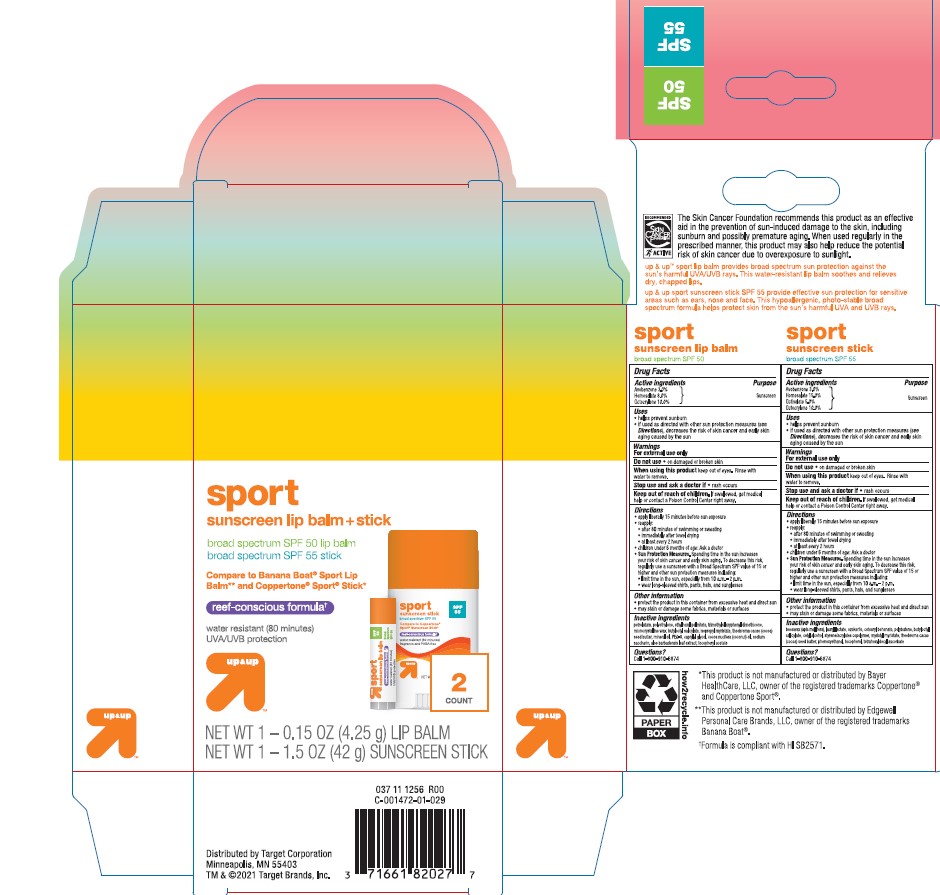
TARGET UP AND UP SPORT SUNSCREEN LIP BALM PLUS STICK- avobenzone, homosalate, octisalate, octocrylene
TARGET CORPORATION
----------
Active ingredients
Avobenzone 3.0%
Homosalate 8.0%
Octocrylene 10.0%
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see
Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Warnings
For external use only.
Do not use
- on damaged or broken skin
When using this product
keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if
Keep out of reach of the children
If product is swallowed, get medical help or contact a Poison Control Center right away
Directions
- apply liberally 15 minutes before sun exposure
- reapply:
- after 80 minutes of swimming or sweating
- immediately after towel drying
- at least every 2 hours
- children under 6 months of age: Ask a doctor
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
Inactive ingredients
Petrolatum, Polyethylene, Ethylhexyl Palmitate, Trimethylsiloxyphenyl Dimethicone, Microcrystalline Wax, Butyloctyl Salicylate, Isopropyl Myristate, Theobroma Cacao (Cocoa) Seed Butter, Mineral Oil, PEG-8, Caprylyl Glycol, Cocos Nucifera (Coconut) Oil, Sodium Saccharin, Aloe Barbadensis Leaf Extract, Tocopheryl Acetate.
Other information
- protect the product in this container from excessive heat and direct sun
- may stain or damage some fabrics, materials, and surfaces
Active ingredient
Avobenzone 3.0%
Homosalate 15.0%
Octisalate 5.0%
Octocrylene 10.0%
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Warnings
For external use only.
Do not use
- on damaged or broken skin
When using this product
keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if
Keep out of reach of the children
If product is swallowed, get medical help or contact a Poison Control Center right away
Directions
- apply liberally 15 minutes before sun exposure
- reapply:
- after 80 minutes of swimming or sweating
- immediately after towel drying
- at least every 2 hours
- children under 6 months of age: Ask a doctor
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
Inactive ingredients
BEESWAX, LAURYL LACTATE, OZOKERITE, CETEARYL BEHENATE, POLYBUTENE, BUTYLOCTYL SALICYLATE, CETYL ALCOHOL, STYRENE/ACRYLATES COPOLYMER, MYRISTYL MYRISTATE, THEOBROMA CACAO (COCOA) SEED BUTTER, PHENOXYETHANOL, TOCOPHEROL, TETRAHEXYLDECYL ASCORBATE.
Other information
- protect the product in this container from excessive heat and direct sun
- may stain or damage some fabrics, materials, and surfaces