Dr. Luke Nail Repair Pen by Dr.luke Healthcare LLC 83179-007-01 Completed
Dr. Luke Nail Repair Pen by
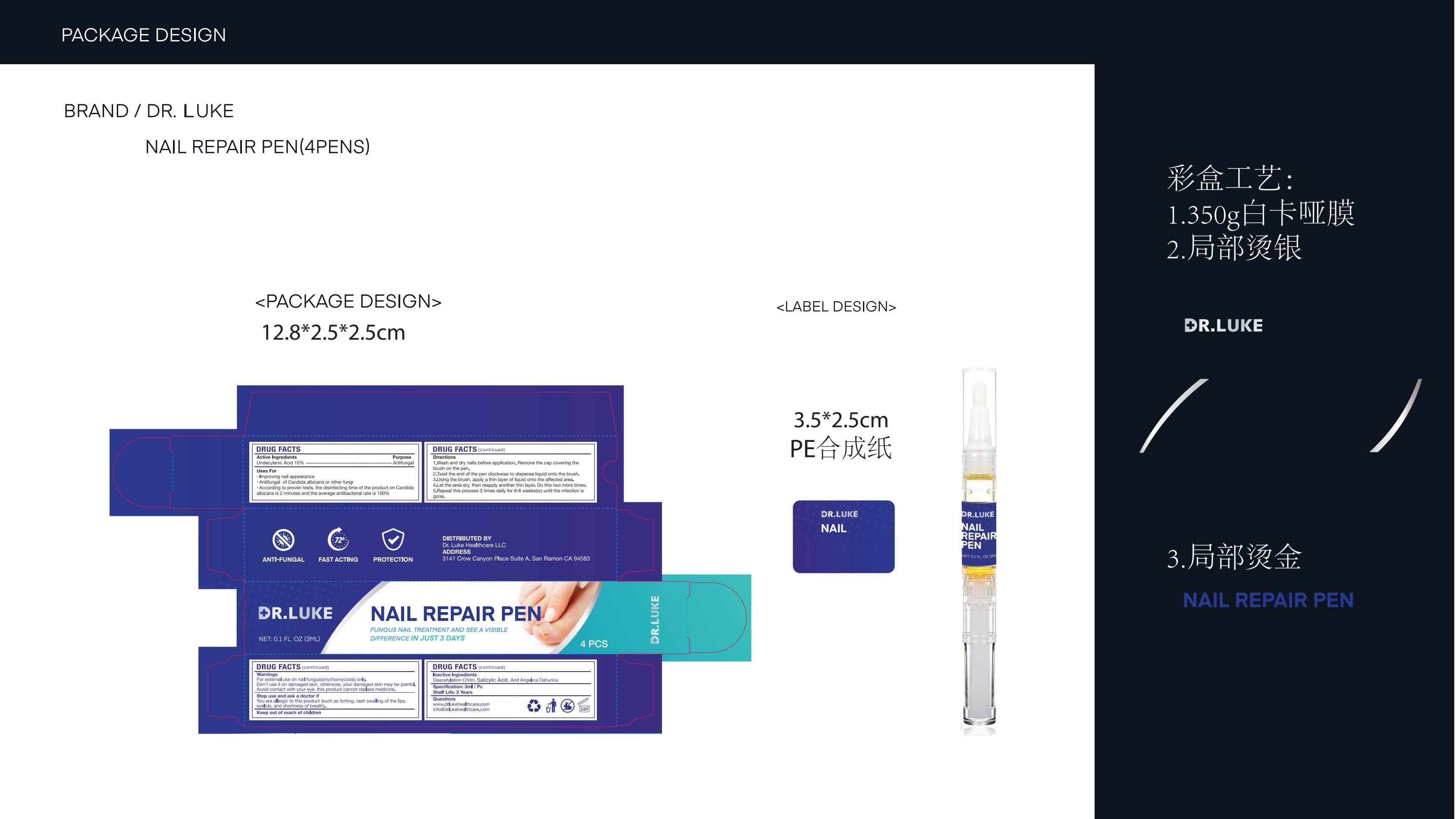
Drug Labeling and Warnings
Dr. Luke Nail Repair Pen by is a Otc medication manufactured, distributed, or labeled by Dr.luke Healthcare LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
DR. LUKE NAIL REPAIR PEN- nail repair pen liquid
Dr.luke Healthcare LLC
----------
83179-007-01 Completed
Use
Improving nail appearance
Antifungal of Candida albicans or other fungi
According to proven tests, the disinfecting time of the product on Candidaalbicans is 2 minutes and the average antibacterial rate is 100%
Warnings
For external use on nailfungus(onychomycosis) only, pleasedon't use it on healthy nails
Don't use it on damaged skin,otherwise, your damaged skin maybe painful.
Avoid contact with your eye. This product cannot replace medicine.
Do not use
On damaged skin(cuts, abrasions, eczema, sunburn).
If you are allergic to any of the ingredients in this product.
If you are pregnant or breastfeeding.
WHEN USING SECTION
Improving nail appearance
Antifungal of Candida albicans or other fungi
According to proven tests, the disinfecting time of the product on Candidaalbicans is 2 minutes and the average antibacterial rate is 100%
STOP USE section
You are allergic to this product (such as itching, rash swelling of the lips.evelids, and shortness of breath)
ASK DOCTOR
You are allergic to this product (such as itching, rash swelling of the lips.evelids, and shortness of breath)
Directions
1.Wash and dry nails before application. Remove the cap covering thebrush on the pen.
2.Twist the end of the pen clockwise to dispense liquid onto the brush.
3.Using the brush, apply a thin layer of liquid onto the affected area.
4.Let the area dry, then reapply another thin layer. Do this two more times.
5.Repeat this process 3 times daily for 6-8 weeks(or) until the infection is gone.
| DR. LUKE NAIL REPAIR PEN
nail repair pen liquid |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Dr.luke Healthcare LLC (118868014) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Dr.luke Healthcare LLC | 118868014 | label(83176-007) , manufacture(83176-007) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.