COLD SPOT POINT RELIEF- menthol, methyl salicylate gel
Cold Spot by
Drug Labeling and Warnings
Cold Spot by is a Otc medication manufactured, distributed, or labeled by Fabrication Enterprises, Pure Source, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- KEEP OUT OF REACH OF CHILDREN
- WARNINGS
- PURPOSE
- INDICATIONS & USAGE
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
COLD SPOT POINT RELIEF
menthol, methyl salicylate gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 51452-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 120 mg in 1 mL METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ARNICA CORDIFOLIA FLOWER (UNII: JCG1OSZ7A8) CARBOMER 1342 (UNII: 809Y72KV36) ILEX PARAGUARIENSIS LEAF (UNII: 1Q953B4O4F) EUCALYPTUS GLOBULUS LEAF (UNII: S546YLW6E6) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) PROPYLPARABEN (UNII: Z8IX2SC1OH) PEPPERMINT OIL (UNII: AV092KU4JH) DIMETHYL SULFONE (UNII: 9H4PO4Z4FT) POLYSORBATE 80 (UNII: 6OZP39ZG8H) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51452-001-32 960 mL in 1 BOTTLE; Type 0: Not a Combination Product 08/24/2010 02/01/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 08/24/2010 02/01/2021 Labeler - Fabrication Enterprises (070577218) Registrant - Fabrication Enterprises (070577218) Establishment Name Address ID/FEI Business Operations Pure Source, LLC 080354456 manufacture(51452-001)
Trademark Results [Cold Spot]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
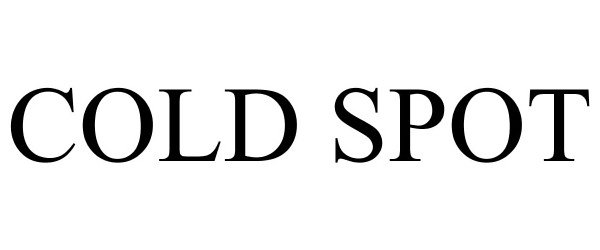 COLD SPOT 78922431 not registered Dead/Abandoned |
Music Performance Laboratory, Inc. 2006-07-05 |
 COLD SPOT 78119312 2681344 Live/Registered |
Beekley Corporation 2002-04-03 |
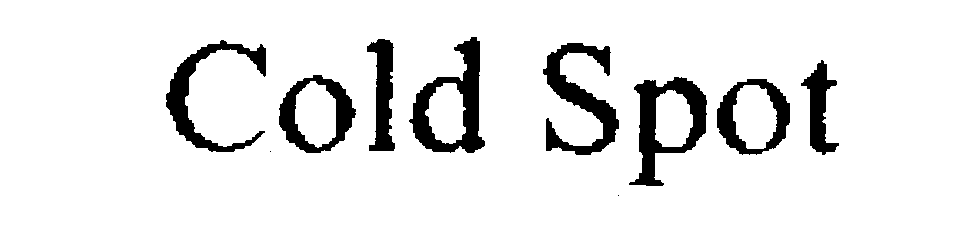 COLD SPOT 76040090 not registered Dead/Abandoned |
Connie Core-Dubay 2000-05-02 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
