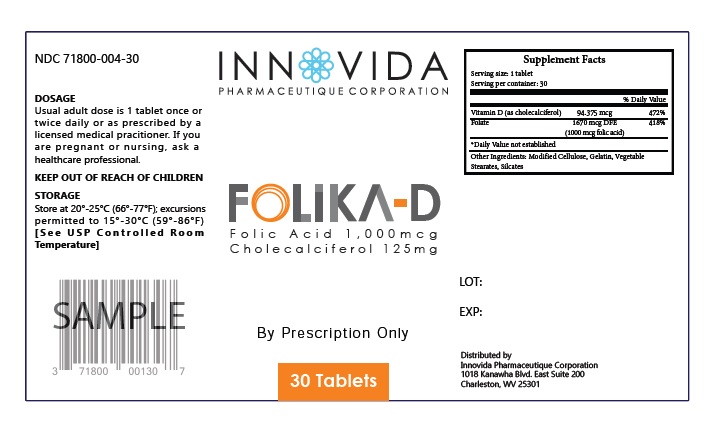
FOLIKA-D by Innovida Phamaceutique Corporation Innovida FOLIKA-D
FOLIKA-D by
Drug Labeling and Warnings
FOLIKA-D by is a Other medication manufactured, distributed, or labeled by Innovida Phamaceutique Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
FOLIKA-D- folic acid, cholecalciferol, folate tablet
Innovida Phamaceutique Corporation
----------
Innovida FOLIKA-D
Usual adult dose is 1 tablet once or twice daily or as prescribed by a licensed medical practitioner. If you are pregnant or nursing, ask a healthcare professional.
This product is contraindicated in patients with a known hypersensitivity to any of the ingredients.
Folika-D tablets should only be used under the direction and supervision of a licensed medical practitioner. Use with caution in patients that may have a medical condition, are pregnant, lactating, trying to conceive, under the age of 18, or taking medications.
Pregnancy and Lactation
Folika-D is not intended for use in pregnant or lactating patients.
| FOLIKA-D
folic acid, cholecalciferol, folate tablet |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Supplement Facts | ||
| Serving Size : | Serving per Container : | |
| Amount Per Serving | % Daily Value | |
|---|---|---|
| scoring | 1 | |
| color | ||
| size (solid drugs) | 5 mm | |
| shape | ||
| Labeler - Innovida Phamaceutique Corporation (080892908) |
| Registrant - Innovida Phamaceutique Corporation (080892908) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Innovida Phamaceutique Corporation | 080892908 | manufacture(71800-004) | |