Retail Hand Sanitizer by Kay Chemical Company Drug Facts
Retail Hand Sanitizer by
Drug Labeling and Warnings
Retail Hand Sanitizer by is a Otc medication manufactured, distributed, or labeled by Kay Chemical Company. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
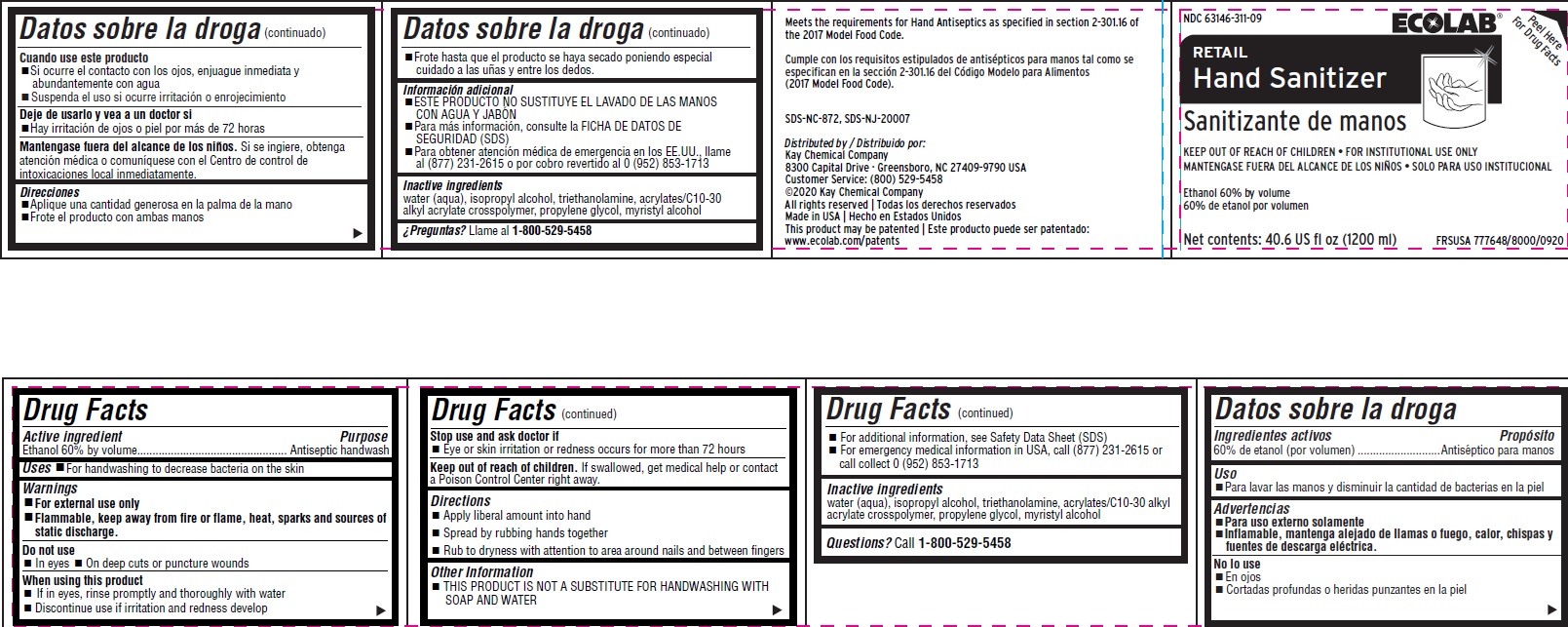
RETAIL HAND SANITIZER- alcohol liquid
Kay Chemical Company
----------
Drug Facts
Warnings
- For external use only
- FLAMMABLE, keep away from fire or flame, heat, sparks and sources of static discharge
Directions
- Apply liberal amount into hand
- Spread by rubbing hands together
- Rub to dryness with attention to are around nails and between fingers
Other information
- For additional information, see Safety Data Sheet (SDS)
- For emergency medical information in USA, call (877) 231-2615 or call collect 0 (952) 853-1713
Inactive ingredients water (aqua), isopropyl alcohol, triethanolamine, acrylates/C10-30 alkyl acrylate crosspolymer, propylene glycol, myristyl alcohol
Principal display panel and representative label
NDC: 63146-311-09
RETAIL
Hand Sanitizer
Sanitizante de manos
KEEP OUT OF REACH OF CHILDREN FOR INSTITUTIONAL USE ONLY
MANTENGASE FUERA DEL ALCANCE DE LOS NIÑOS SOLO PARA USO INSTITUCIONAL
Ethanol 60% by volume
60% de etanol por volume
Net contents: 40.6 US fl oz (1200 ml)
FRSUSA 777648/8000/0920
Meets the requirements for Hand Antiseptics as specified in section 2-301.16 of the 2017 Model Food Code.
Cumple con los requisitos estipulados de antisépticos para manos tal como se especifican en la sección 2-301.16 del Código Modelo para Alimentos
(2017 Model Food Code).
SDS-NC-872, SDS-NJ-20007
Distributed by / Distribuido por:
Kay Chemical Company
8300 Capital Drive · Greensboro, NC 27409-9790 USA
Customer Service: (800) 529-5458
©2020 Kay Chemical Company
All rights reserved | Todas los derechos reservados
Made in USA | Hecho en Estados Unidos
This product may be patented | Este producto puede ser patentado:
| RETAIL HAND SANITIZER
alcohol liquid |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Labeler - Kay Chemical Company (003237021) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.