HealthyDerm Molluscum Contagiosum Treatment Oil by Shenzhen Situya Trading Co., Ltd. 82739-008 Complete
HealthyDerm Molluscum Contagiosum Treatment Oil by
Drug Labeling and Warnings
HealthyDerm Molluscum Contagiosum Treatment Oil by is a Otc medication manufactured, distributed, or labeled by Shenzhen Situya Trading Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
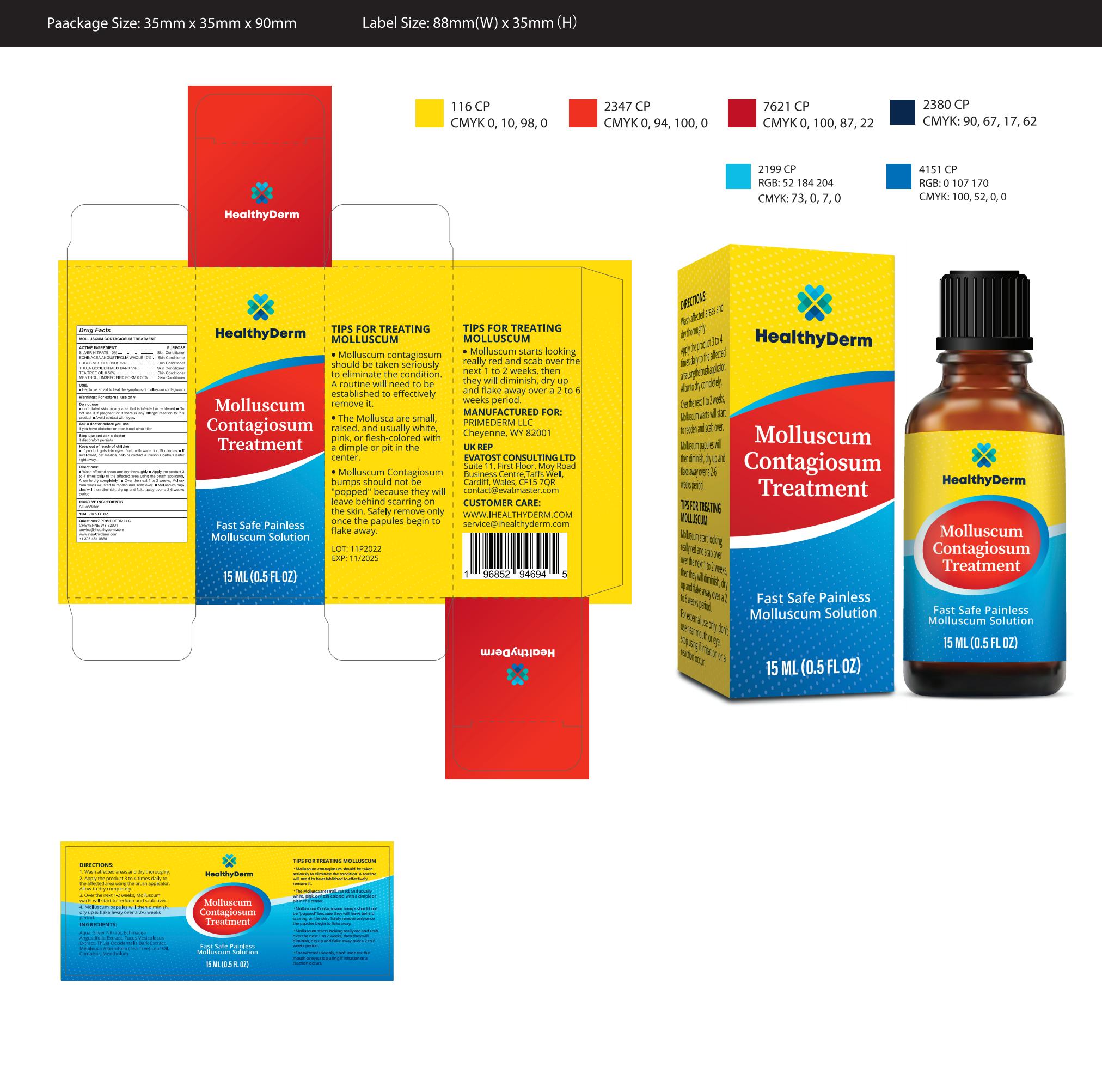
HEALTHYDERM MOLLUSCUM CONTAGIOSUM TREATMENT OIL- molluscum contagiosum treatment oil
Shenzhen Situya Trading Co., Ltd.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
82739-008 Complete
Active Ingredient
SILVER NITRATE 10%
ECHINACEAANGUSTIFOLIA WHOLE 10%
FUCUS VESICULOSUS 5%
THUJA OCCIDENTALIS BARK 5%
TEA TREE OIL 0.50%
MENTHOL,UNSPECIFIED FORM 0.50%
Do not use
On irritated skin on any area that is infected or reddened
Do not use it if pregnant or if there is any allergic reaction to thisproduct
Avoid contact with eyes.
KEEP OUT OF REACH OF CHILDREN
lf product gets into eyes, flush with water for 15 minutes
lf swallowed, get medical help or contact a Poison Control Centerright away.
Directions
Wash affected areas and dry thoroughly.
Apply the product 3to 4 times daily to the affected area using the brush applicator.Allow to dry completely.
Over the next 1 to 2 weeks, Mollus-cum warts will start to redden and scab over.
Molluscum pap-ules will then diminish, dry up and flake away over a 2-6 weeksperiod.
| HEALTHYDERM MOLLUSCUM CONTAGIOSUM TREATMENT OIL
molluscum contagiosum treatment oil |
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
| Labeler - Shenzhen Situya Trading Co., Ltd. (706154255) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Shenzhen Situya Trading Co., Ltd. | 706154255 | manufacture(82739-008) | |