OMNIPAQUE- iohexol injection, solution
OMNIPAQUE by
Drug Labeling and Warnings
OMNIPAQUE by is a Prescription medication manufactured, distributed, or labeled by GE Healthcare Inc., GE Healthcare Shanghai, Co., Ltd., GE Healthcare Ireland Limited, GE Healthcare Lindesnes, GE Healthcare AS. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use OMNIPAQUE safely and effectively. See full prescribing information for OMNIPAQUE.
OMNIPAQUE (iohexol) injection, for intrathecal, intravascular, oral, rectal, intraarticular, or body cavity use.
OMNIPAQUE (iohexol) oral solution
Initial U.S. Approval: 1985WARNING: RISKS WITH INADVERTENT INTRATHECAL ADMINISTRATION OF OMNIPAQUE injection 140 and 350 mg iodine/mL.
See full prescribing information for complete boxed warning.
Inadvertent intrathecal administration may cause death, convulsions/seizures, cerebral hemorrhage, coma, paralysis, arachnoiditis, acute renal failure, cardiac arrest, rhabdomyolysis, hyperthermia, and brain edema (4, 5.1).
INDICATIONS AND USAGE
OMNIPAQUE (iohexol) injection is a radiographic contrast agent indicated for intrathecal, intravascular, oral, rectal, intraarticular and body cavity use. OMNIPAQUE oral solution is indicated for oral use only in conjunction with OMNIPAQUE injection administered intravenously for computed tomography (CT) of the abdomen (1).
DOSAGE AND ADMINISTRATION
The concentration and volume required will depend on the indication, size and condition of the patient, and the equipment and imaging technique used. See full prescribing information for complete dosing information (2).
DOSAGE FORMS AND STRENGTHS
OMNIPAQUE Injection (3)
- 140 mg of iodine per mL (302 mg of iohexol/mL) in +PlusPak™ polymer bottles
- 180 mg of iodine per mL (388 mg of iohexol/mL) in glass vials
- 240 mg of iodine per mL (518 mg of iohexol/mL), 300 mg of iodine per mL (647 mg of iohexol/mL) and 350 mg of iodine per mL (755 mg of iohexol/mL) in glass vials and bottles and +PlusPak™ polymer bottles
OMNIPAQUE Oral Solution (3)
- 9 mg of iodine per mL (19 mg of iohexol/mL) and 12 mg of iodine per mL (26 mg of iohexol/mL) in +PlusPak™ polymer bottles
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Hypersensitivity Reactions: Life-threatening or fatal reactions can occur. Always have emergency equipment and trained personnel available. (5.3)
- Contrast Induced Acute Kidney Injury: Acute injury including renal failure can occur. Minimize dose and maintain adequate hydration to minimize risk. (5.4)
- Cardiovascular Reactions: Hemodynamic disturbances including shock and cardiac arrest may occur during or after administration. (5.5)
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥ 1.0%) in adult patients after OMNIPAQUE administration. (6.1).
- Intrathecal: Headaches, Pain including backache, neckache, stiffness and neuralgia, nausea, vomiting and dizziness
- Intravascular: Pain, vision abnormalities (including blurred vision and photomas), headache, taste perversion, arrhythmias including premature ventricular contractions (PVCs) and premature atrial contractions (PACs), angina/chest pain, nausea
- Oral: Diarrhea, nausea, vomiting, abdominal pain, flatulence, headache
- Body Cavity: Pain, swelling and heat sensation
Post-marketing adverse reactions (6.2): Hypersensitivity and manifestations like rash, pruritus, urticaria, and dyspnea, in addition chest pain, and swelling.
To report SUSPECTED ADVERSE REACTIONS, contact GE Healthcare at 1-800-654-0118 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
- Lactation: A lactating woman may pump and discard breast milk for 10 hours after OMNIPAQUE administration. (8.2)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 4/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: RISKS WITH INADVERTENT INTRATHECAL ADMINISTRATION
OMNIPAQUE injection, 140 and 350 mg iodine/mL.
See full prescribing information for complete boxed warning1 INDICATIONS AND USAGE
1.1 Intrathecal Administration
1.2 Intravascular Administration
1.3 Oral or Rectal Administration
1.4 Oral Administration in Conjunction with Intravenous Administration
1.5 Intraarticular Administration
1.6 Body Cavity Administration
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Instructions
2.2 Intrathecal Dosage and Administration
2.3 Intravascular Dosage and Administration
2.4 Oral or Rectal Dosage and Administration
2.5 Oral Dosage and Administration in Conjunction with Intravenous Administration
2.6 Intraarticular Dosage and Administration
2.7 Body Cavity Dosage and Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risks Associated with Inadvertent Intrathecal Administration
5.2 Risks Associated with Inadvertent Parenteral Administration of Omnipaque Oral Solution
5.3 Hypersensitivity Reactions
5.4 Contrast Induced Acute Kidney Injury
5.5 Cardiovascular Adverse Reactions
5.6 Thromboembolic Events
5.7 Extravasation and Injection Site Reactions
5.8 Thyroid Storm in Patients with Hyperthyroidism
5.9 Hypertensive Crisis in Patients with Pheochromocytoma
5.10 Sickle Cell Crisis in Patients with Sickle Cell Disease
5.11 Severe Cutaneous Adverse Reactions
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Post-marketing Experience
7 DRUG INTERACTIONS
7.1 Drug-Drug Interactions
7.2 Drug Laboratory Test Interactions
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
10.1 Intravascular Administration
11 DESCRIPTION
11.1 Chemical Characteristics
11.2 Physical Characteristics
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: RISKS WITH INADVERTENT INTRATHECAL ADMINISTRATION
OMNIPAQUE injection, 140 and 350 mg iodine/mL.
See full prescribing information for complete boxed warningInadvertent intrathecal administration may cause death, convulsions/seizures, cerebral hemorrhage, coma, paralysis, arachnoiditis, acute renal failure, cardiac arrest, rhabdomyolysis, hyperthermia, and brain edema (4, 5.1)
-
1 INDICATIONS AND USAGE
1.1 Intrathecal Administration
1.2 Intravascular Administration
Adults
OMNIPAQUE 140
- Intra-arterial digital subtraction angiography of the head, neck, abdominal, renal and peripheral vessels.
OMNIPAQUE 240
- CT head imaging
- Peripheral venography (phlebography).
OMNIPAQUE 300
- Aortography including studies of the aortic arch, abdominal aorta and its branches
- CT head and body imaging
- Cerebral arteriography
- Peripheral venography (phlebography)
- Peripheral arteriography
- Excretory urography.
OMNIPAQUE 350
- Angiocardiography (ventriculography, selective coronary arteriography)
- Aortography including studies of the aortic root, aortic arch, ascending aorta, abdominal aorta and its branches
- CT head and body imaging
- Intravenous digital subtraction angiography of the head, neck, abdominal, renal and peripheral vessels
- Peripheral arteriography
- Excretory urography.
Pediatrics
OMNIPAQUE 240
- CT head and body imaging.
OMNIPAQUE 300
- Angiocardiography (ventriculography)
- Excretory urography
- CT head and body imaging.
OMNIPAQUE 350
- Angiocardiography (ventriculography, pulmonary arteriography, venography, and studies of the collateral arteries)
- Aortography including the aortic root, aortic arch, ascending and descending aorta.
1.4 Oral Administration in Conjunction with Intravenous Administration
Diluted OMNIPAQUE Injection
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Instructions
- OMNIPAQUE 140, 180, 240, 300 and 350 are indicated for intravascular, oral, rectal, intraarticular, and body cavity administration. OMNIPAQUE 180, 240, and 300 are indicated for intrathecal administration [see Boxed Warning, Contraindications (4), Warnings and Precautions (5.1)].
- Use sterile technique for all handling and administration of OMNIPAQUE for intravascular, intrathecal, intraarticular, and body cavity administration.
- OMNIPAQUE oral solution 9 and 12 are indicated for oral use only [see Contraindications (4) and Warnings and Precautions (5.2)].
- Do not use if tamper-evident ring is broken or missing.
- OMNIPAQUE injection may be administered at either body (37°C) or room temperature.
- Inspect OMNIPAQUE injection for particulate matter or discoloration before administration, whenever solution and container permit. Do not administer if OMNIPAQUE injection contains particulate matter or is discolored.
- Do not mix OMNIPAQUE injection with, or inject in intravenous lines containing, other drugs or total nutritional admixtures.
- Use the lowest dose necessary to obtain adequate visualization.
- Individualize the volume, strength, and rate of administration of OMNIPAQUE injection. Consider factors such as age, body weight, vessel size, blood flow rate within the vessel, anticipated pathology, degree and extent of opacification required, structures or area to be examined, disease processes affecting the patient, and equipment and technique to be employed.
- Avoid extravasation when administering OMNIPAQUE injection intravascularly, especially in patients with severe arterial or venous disease [see Warnings and Precautions (5.6)].
- Hydrate patients before and after intravascular administration of OMNIPAQUE injection [see Warnings and Precautions (5.4)].
- Each bottle of OMNIPAQUE injection and oral solution is intended for one procedure only. Discard any unused portion.
2.2 Intrathecal Dosage and Administration
- Rate of injection: Injection should be made slowly over 1 to 2 minutes
- Repeat procedures: If sequential or repeat examinations are required, a suitable interval of time between administrations should be observed to allow for normal clearance of the drug from the body; at least 48 hours should be allowed before repeat examination; however, whenever possible, 5 to 7 days is recommended.
- If computerized tomographic (CT) myelography follows myelography, delay imaging several hours to allow the degree of contrast to decrease.
TABLE 1 - INTRATHECAL ADULTS The usual recommended total doses for use in lumbar, thoracic, cervical, and total columnar myelography in adults are 1,200 mg iodine to 3,100 mg iodine (see below). STUDY TYPE INJECTION TYPE CONCENTRATION
(mg iodine/mL)VOLUME
(mL)* A total dose of 3,100 mg iodine or a concentration of 300 mg iodine/mL should not be exceeded in adults. LUMBAR MYELOGRAPHY LUMBAR OMNIPAQUE 180
OMNIPAQUE 24010 to 17
7 to 12.5THORACIC MYELOGRAPHY LUMBAR
CERVICALOMNIPAQUE 240
OMNIPAQUE 3006 to 12.5
6 to 10CERVICAL MYELOGRAPHY LUMBAR OMNIPAQUE 240
OMNIPAQUE 3006 to 12.5
6 to 10CERVICAL MYELOGRAPHY C1-2 OMNIPAQUE 180
OMNIPAQUE 240
OMNIPAQUE 3007 to 10
6 to 12.5
4 to 10TOTAL COLUMNAR MYELOGRAPHY LUMBAR OMNIPAQUE 240
OMNIPAQUE 3006 to 12.5
6 to 10TABLE 2 – INTRATHECAL PEDIATRICS The usual recommended total doses for lumbar, thoracic, cervical, and/or total columnar myelography by lumbar puncture in children are 360 mg iodine to 2700 mg iodine (see below). Actual volumes administered depend largely on patient age and the following guidelines are recommended. AGE STUDY TYPE INJECTION TYPE CONCENTRATION
(mg iodine/mL)VOLUME
(mL)*A total dose of 2,700 mg iodine or a concentration of 180 mg iodine/mL should not be exceeded in a single myelographic examination in pediatrics. 0 up to 3 mos. LUMBAR, THORACIC, CERVICAL AND/OR TOTAL COLUMNAR MYELOGRAPHY LUMBAR PUNCTURE OMNIPAQUE 180 2 to 4 3 up to 36 mos. OMNIPAQUE 180 4 to 8 3 up to 7 yrs. OMNIPAQUE 180 5 to 10 7 up to 13 yrs. OMNIPAQUE 180 5 to 12 13 to 18 yrs. OMNIPAQUE 180 6 to 15 2.3 Intravascular Dosage and Administration
Intra-arterial Procedures
TABLE 3 ANGIOCARDIOGRAPHIC PROCEDURES PATIENT POPULATION CONCENTRATION
(mg iodine/mL)VOLUME
(mL)Adults OMNIPAQUE 350 VENTRICULOGRAPHY - The recommended single dose is 40 mL (Range of 30 mL to 60 mL)
- May be combined with selective coronary arteriography
- The recommended single dose is 5 mL (Range of 3 mL to 14 mL)
Maximum volume with multiple injections should not exceed 250 mL.Pediatrics OMNIPAQUE 300 VENTRICULOGRAPHY
The recommended single dose is 1.75 mL/kg (Range of 1.5 mL/kg to 2 mL/kg)- May be repeated as necessary
OMNIPAQUE 350 VENTRICULOGRAPHY
Recommended single dose is 1.25 mL/kg (Range of 1 mL/kg to 1.5 mL/kg).- May be repeated as necessary
PULMONARY ANGIOGRAPHY (PULMONARY ARTERIOGRAPHY AND/OR PULMONARY VENOGRAPHY)
The recommended single dose is 1 mL/kg.TABLE 4 AORTOGRAPHY PATIENT POPULATION CONCENTRATION
(mg iodine/mL)VOLUME
(mL)Adults OMNIPAQUE 300 and 350 AORTOGRAPHY AND SELECTIVE VISCERAL ARTERIOGRAPHY
The recommended single dose is:- 50 mL to 80 mL for the aorta (aortic arch, ascending aorta)
- 30 mL to 60 mL for abdominal aorta and its branches (celiac, mesenteric, hepatic and splenic arteries)
- 5 mL to 15 mL for renal arteries
- 290 mL of OMNIPAQUE 300
- 250 mL of OMNIPAQUE 350
OMNIPAQUE 350 AORTIC ROOT AND ARCH STUDY WHEN USED ALONE
The recommended single dose is 50 mL (Range of 20 mL to 75 mL)Pediatrics OMNIPAQUE 350 AORTOGRAPHY (AORTIC ROOT, AORTIC ARCH, AND DESCENDING AORTA)
The recommended single dose is 1 mL/kg.- May be repeated as necessary
TABLE 5 CEREBRAL ARTERIOGRAPHY PATIENT POPULATION CONCENTRATION
(mg iodine/mL)VOLUME
(mL)Adults OMNIPAQUE 300 Single dose for cerebral arteriography is as follows: - Common carotid artery (6 mL to 12 mL)
- Internal carotid artery (8 mL to 10 mL)
- External carotid artery (6 mL to 9 mL)
- Vertebral artery (6 mL to 10 mL)
TABLE 6 INTRA-ARTERIAL DIGITAL SUBTRACTION ANGIOGRAPHY HEAD, NECK, ABDOMINAL, RENAL AND PERIPHERAL VESSELS PATIENT POPULATION CONCENTRATION
(mg iodine/mL)VOLUME
(mL)Mechanical or hand injection can be used to administer one or more bolus intra-arterial injections of OMNIPAQUE 140. Adults OMNIPAQUE 140 ARTERIES VOLUME/INJECTION (mL) RATE OF INJECTION
(mL/sec)Aorta 20 to 45 8 to 20 Carotid 5 to 10 3 to 6 Femoral 9 to 20 3 to 6 Vertebral 4 to 10 2 to 8 Renal 6 to 12 3 to 6 Other branches of aorta (includes subclavian, axillary, innominate and iliac) 8 to 25 3 to 10 TABLE 7 PERIPHERAL ARTERIOGRAPHY PATIENT POPULATION CONCENTRATION
(mg iodine/mL)VOLUME
(mL)Adults OMNIPAQUE 300 and 350 The recommended dose for use in peripheral angiography is as follows:
Aortofemoral runoffs:- 30 mL to 90 mL of OMNIPAQUE 300
- 20 mL to 70 mL of OMNIPAQUE 350
- 10 mL to 60 mL of OMNIPAQUE 300
- 10 mL to 30 mL of OMNIPAQUE 350
Intravenous Procedures
TABLE 8 PERIPHERAL VENOGRAPHY (PHLEBOGRAPHY) PATIENT POPULATION CONCENTRATION
(mg iodine/mL)VOLUME
(mL)Adults OMNIPAQUE 240 and 300 The recommended dose (per leg) is: - 20 mL to 150 mL of OMNIPAQUE 240
- 40 mL to 100 mL of OMNIPAQUE 300
TABLE 9 EXCRETORY UROGRAPHY PATIENT POPULATION CONCENTRATION
(mg iodine/mL)VOLUME
(mL)Adults OMNIPAQUE 300 and 350 The recommended dose is: - 0.6 mL/kg to 1.2 mL/kg body weight
Pediatrics OMNIPAQUE 300 Dose ranging from 0.5 mL/kg to 3 mL/kg of body weight: - The usual dose for children is 1 mL/kg to 1.5 mL/kg.
- The total administered dose should not exceed 3 mL/kg.
TABLE 10 DIGITAL SUBTRACTION ANGIOGRAPHY HEAD, NECK, ABDOMINAL, RENAL AND PERIPHERAL VESSELS PATIENT POPULATION CONCENTRATION
(mg iodine/mL)VOLUME
(mL)RATE OF INJECTION
(mL/sec)Adults OMNIPAQUE 350 The usual dose for the intravenous digital technique is 30 mL to 50 mL.
Frequently three or more doses may be required, up to a total volume not to exceed 250 mL7.5 mL/second to 30 mL/second using a pressure injector TABLE 11 CT SCANNING OF THE HEAD AND BODY PATIENT POPULATION CONCENTRATION
(mg iodine/mL)VOLUME
(mL)Adults OMNIPAQUE 240, 300 and 350 Head and body imaging by rapid injection
CT Imaging – Head:- 70 mL to 150 mL of OMNIPAQUE 300
- 80 mL of OMNIPAQUE 350
CT Imaging – Body:- 50 mL to 200 mL of OMNIPAQUE 300
- 60 mL to 100 mL of OMNIPAQUE 350
CT Imaging – Head:- 120 mL to 250 mL of OMNIPAQUE 240
Pediatrics OMNIPAQUE 240 and 300
CT Imaging – Head and Body: - 1 mL/kg to 2 mL/kg (with maximum = 3 mL/kg)
- Maximum single dose = 116 mL
2.4 Oral or Rectal Dosage and Administration
Oral and Rectal Administration – Undiluted OMNIPAQUE Injection for Radiographic Examination of the Gastrointestinal (GI) Tract
TABLE 12 DOSING FOR RADIOGRAPHIC EXAMINATION OF THE GI TRACT PATIENT POPULATION CONCENTRATION
(mg iodine/mL)ORAL VOLUME
(mL)RECTAL VOLUME*
(mL)- * When given rectally, larger volumes may be used.
Adults OMNIPAQUE 350 The recommended dose is 50 mL to 100 mL - Pediatrics OMNIPAQUE 180, 240 and 300 The recommended dose is 5 mL to 100 mL The recommended dose is 5 mL to 100 mL* Less than 3 months old OMNIPAQUE 180 5 mL to 30 mL -* Three months to 3 years OMNIPAQUE 180, 240 and 300 Up to 60 mL -* Four years to 10 years OMNIPAQUE 180, 240 and 300 Up to 80 mL -* Greater than 10 years Up to 100 mL -* 2.5 Oral Dosage and Administration in Conjunction with Intravenous Administration
See Table 16 for concurrent intravenous dosing.
Oral Administration of Diluted OMNIPAQUE Injection in Conjunction with Intravenous Administration of OMNIPAQUE Injection for CT of the Abdomen
TABLE 13 DOSING OF DILUTED* OMNIPAQUE INJECTION FOR ORAL ADMINISTRATION PATIENT POPULATION ORAL CONCENTRATION
(mg iodine/mL)ORAL VOLUME
(mL)ADMINISTRATION INSTRUCTIONS - * Dilutions of OMNIPAQUE should be prepared just prior to use and any unused portion discarded after the procedure.
Adults OMNIPAQUE 240, 300 and 350 DILUTED to 6 to 12 mg iodine/mL
(See Table 14 below)Recommended oral dose is: - 500 mL to 1000 mL.
Smaller administered volumes can be given if the iodine concentration in final diluted product is increased
(See Table 14 below)
The oral dosage may be given all at once or over a period of up to 45 minutes if there is difficulty in consuming the required volume.Pediatrics OMNIPAQUE 240, 300 and 350 DILUTED to 9 to 21 mg iodine/mL
(See Table 14 below)Recommended oral dose is: - 180 mL to 750 mL.
Do not exceed an oral dose of 10 grams iodine for patients 3 to 18 years old.Smaller administered volumes can be given if the iodine concentration in final diluted product is increased
(See Table 14 below)
The oral dosage may be given all at once or over a period of up to 45 minutes if there is difficulty in consuming the required volume.TABLE 14 PROCEDURE FOR PREPARATION OF DILUTED OMNIPAQUE INJECTION FOR ORAL ADMINISTRATION OMNIPAQUE to be mixed with liquid such as water, carbonated beverage, milk, infant formula, or juice to achieve one liter of oral contrast agent. Final Iodine Concentration of Diluted Contrast Agent
(mg iodine/mL)OMNIPAQUE 240 OMNIPAQUE 300 OMNIPAQUE 350 Volume of Contrast Agent (mL) Volume of Liquid (mL) Volume of Contrast Agent (mL) Volume of Liquid (mL) Volume of Contrast Agent (mL) Volume of Liquid (mL) 6 25 975 20 980 17 983 9 38 962 30 970 26 974 12 50 950 40 960 35 965 15 63 937 50 950 43 957 18 75 925 60 940 52 948 21 88 912 70 930 60 940 Oral Administration of OMNIPAQUE Oral Solution in Conjunction with Intravenous Administration of OMNIPAQUE Injection for CT of the Abdomen
TABLE 15 DOSING AND ADMINISTRATION OF OMNIPAQUE ORAL SOLUTION PATIENT POPULATION ORAL CONCENTRATION
(mg iodine/mL)ORAL VOLUME
(mL)ADMINISTRATION INSTRUCTIONS Adults OMNIPAQUE oral solution 9 and 12 The recommended oral dose is: - 500 mL to 1000 mL
The oral dosage may be given all at once or over a period of up to 45 minutes if there is difficulty in consuming the required volume. Pediatrics OMNIPAQUE oral solution 9 and 12 The recommended oral dose is: - 180 mL to 750 mL
Do not exceed an oral dose of 10 grams iodine for patients 3 to 18 years old.The oral dosage may be given all at once or over a period of up to 45 minutes if there is difficulty in consuming the required volume. TABLE 16 INTRAVENOUS ADMINISTRATION OF OMNIPAQUE INJECTION FOR CT OF THE ABDOMEN IN CONJUNCTION WITH ORALLY ADMINISTERED DILUTED OMNIPAQUE INJECTION OR OMNIPAQUE ORAL SOLUTION PATIENT POPULATION INTRAVENOUS CONCENTRATION
(mg iodine/mL)INTRAVENOUS VOLUME
(mL)ADMINISTRATION INSTRUCTIONS Adults OMNIPAQUE 300 The recommended dose is: - 100 to 150 mL
Administer up to 40 minutes AFTER consumption of the oral dose Pediatrics OMNIPAQUE 240 and 300 The recommended dose is: - 2 mL/kg with a range of 1 mL/kg to 2 mL/kg (maximum 3 mL/kg)
Administer up to 60 minutes AFTER consumption of the oral dose 2.6 Intraarticular Dosage and Administration
TABLE 17 ARTHROGRAPHY PATIENT POPULATION LOCATION CONCENTRATION
(mg iodine/mL)VOLUME
(mL)DOUBLE CONTRAST/SINGLE CONTRAST - * Passive or active manipulation is used to disperse the medium throughout the joint space.
Adults Knee* OMNIPAQUE 240 5 to 15 Lower volumes recommended for double-contrast examinations; higher volumes recommended for single-contrast examinations. OMNIPAQUE 300 5 to 15 OMNIPAQUE 350 5 to 10 Adults Shoulder* OMNIPAQUE 240 3 OMNIPAQUE 300 10 Adults Temporomandibular* OMNIPAQUE 300 0.5 to 1 2.7 Body Cavity Dosage and Administration
Body Cavity Administration - Undiluted OMNIPAQUE Injection
TABLE 18 ENDOSCOPIC RETROGRADE PANCREATOGRAPHY (ERP) ENDOSCOPIC RETROGRADE CHOLANGIOPANCREATOGRAPHY (ECRP) PATIENT POPULATION CONCENTRATION
(mg iodine/mL)VOLUME
(mL)Adults OMNIPAQUE 240 10 mL to 50 mL but may vary depending on individual anatomy and/or disease state. TABLE 19 HYSTEROSALPINGOGRAPHY PATIENT POPULATION CONCENTRATION
(mg iodine/mL)VOLUME
(mL)Adults OMNIPAQUE 240 and 300 15 mL to 20 mL but may vary depending on individual anatomy and/or disease state. TABLE 20 HERNIOGRAPHY PATIENT POPULATION CONCENTRATION
(mg iodine/mL)VOLUME
(mL)Adults OMNIPAQUE 240 50 mL but may vary depending on individual anatomy and/or disease state. Body Cavity Administration - Diluted OMNIPAQUE Injection
TABLE 21 VOIDING CYSTOURETHROGRAPHY (VCU) (CAN BE PERFORMED IN CONJUNCTION WITH EXCRETORY UROGRAPHY) PATIENT POPULATION CONCENTRATION
(mg iodine/mL)VOLUME
(mL)Pediatrics The concentration may vary depending upon the patient's size and age and with the technique and equipment used.
OMNIPAQUE injection may be diluted with Sterile Water for Injection.
(See Table 22 below).OMNIPAQUE injection may be diluted, utilizing aseptic technique, with Sterile Water for Injection to a concentration of 50 mg iodine/mL to 100 mg iodine/mL for voiding cystourethrography.
Range:- 50 mL to 300 mL of DILUTED OMNIPAQUE at a concentration of 100 mg iodine/mL
- 50 mL to 600 mL of DILUTED OMNIPAQUE at a concentration of 50 mg iodine/mL.
TABLE 22 PROCEDURE FOR PREPARATION OF DILUTED* OMNIPAQUE INJECTION FOR VCU Final iodine Concentration of Diluted Contrast Agent
(mg iodine/mL)Volume of OMNIPAQUE 240
(mL)Volume of Sterile Water for Injection
(mL)Volume of OMNIPAQUE 300
(mL)Volume of Sterile Water for Injection (mL) Volume of OMNIPAQUE 350
(mL)Volume of Sterile Water for Injection (mL) - * Dilutions of OMNIPAQUE should be prepared just prior to use and any unused portion discarded after the procedure.
100 100 140 100 200 100 250 90 167 233 289 80 200 275 338 70 243 330 400 60 300 400 483 50 380 500 600 -
3 DOSAGE FORMS AND STRENGTHS
OMNIPAQUE (iohexol) Injection and Oral Solution
Sterile, pyrogen-free, gluten-free, colorless to pale yellow solution containing the nonionic, water-soluble x-ray contrast medium iohexol, and available in the following strengths and formats:
OMNIPAQUE (iohexol) Injection
- 140 mg of organically bound iodine per mL (302 mg iohexol/mL)
- Available in +PLUSPAK™ (polymer bottle)
- 180 mg of organically bound iodine per mL (388 mg iohexol/mL)
- Available in glass vials
- 240 mg of organically bound iodine per mL (518 mg iohexol/mL)
- 300 mg of organically bound iodine per mL (647 mg iohexol/mL)
- 350 mg of organically bound iodine per mL (755 mg iohexol/mL)
- Available in glass vials and bottles and +PLUSPAK™ polymer bottles.
- 140 mg of organically bound iodine per mL (302 mg iohexol/mL)
-
4 CONTRAINDICATIONS
- OMNIPAQUE 140 and OMNIPAQUE 350 are contraindicated for intrathecal use [see Warnings and Precautions (5.1)]:
- OMNIPAQUE oral solution 9 and 12 are contraindicated for parenteral administration [see Warnings and Precautions (5.2)]:
-
5 WARNINGS AND PRECAUTIONS
5.1 Risks Associated with Inadvertent Intrathecal Administration
OMNIPAQUE injection 140 and 350 are contraindicated for intrathecal use [see Contraindications (4) and Dosage and Administration (2.1)]. Inadvertent intrathecal administration can cause death, convulsions/seizures, cerebral hemorrhage, coma, paralysis, arachnoiditis, acute renal failure, cardiac arrest, rhabdomyolysis, hyperthermia, and brain edema.
5.2 Risks Associated with Inadvertent Parenteral Administration of Omnipaque Oral Solution
OMNIPAQUE oral solution 9 and 12 are contraindicated for parenteral administration [see Contraindications (4) and Dosage and Administration (2.1)]. Adverse reactions such as hemolysis may occur if administered intravascularly. Do not administer OMNIPAQUE oral solution 9 and 12 parenterally.
5.3 Hypersensitivity Reactions
OMNIPAQUE can cause life-threatening or fatal hypersensitivity reactions including anaphylaxis. Manifestations include respiratory arrest, laryngospasm, bronchospasm, angioedema, and shock. Most severe reactions develop shortly after the start of the injection (within 3 minutes), but reactions can occur up to hours later. There is an increased risk in patients with a history of a previous reaction to contrast agent, and known allergies (i.e., bronchial asthma, drug, or food allergies) or other hypersensitivities. Premedication with antihistamines or corticosteroids does not prevent serious life-threatening reactions, but may reduce both their incidence and severity.
Obtain a history of allergy, hypersensitivity, or hypersensitivity reactions to iodinated contrast agents and always have emergency resuscitation equipment and trained personnel available prior to OMNIPAQUE administration. Monitor all patients for hypersensitivity reactions.
5.4 Contrast Induced Acute Kidney Injury
Acute kidney injury, including renal failure, may occur after parenteral administration of OMNIPAQUE. Risk factors include: pre-existing renal impairment, dehydration, diabetes mellitus, congestive heart failure, advanced vascular disease, elderly age, concomitant use of nephrotoxic or diuretic medications, multiple myeloma / paraproteinaceous diseases, repetitive and/or large doses of an iodinated contrast agent.
Use the lowest necessary dose of OMNIPAQUE in patients with renal impairment. Adequately hydrate patients prior to and following parenteral administration of OMNIPAQUE. Do not use laxatives, diuretics, or preparatory dehydration prior to OMNIPAQUE administration.
5.5 Cardiovascular Adverse Reactions
Life-threatening or fatal cardiovascular reactions including hypotension, shock, cardiac arrest have occurred with the parenteral administration of OMNIPAQUE. Most deaths occur during injection or five to ten minutes later, with cardiovascular disease as the main aggravating factor. Cardiac decompensation, serious arrhythmias, and myocardial ischemia or infarction can occur during coronary arteriography and ventriculography.
Based upon clinical literature reported deaths from the administration of iodinated contrast agents range from 6.6 per million (0.00066%) to 1 in 10,000 (0.01%). Use the lowest necessary dose of OMNIPAQUE in patients with congestive heart failure and always have emergency resuscitation equipment and trained personnel available. Monitor all patients for severe cardiovascular reactions.
5.6 Thromboembolic Events
Angiocardiography
Serious, rarely fatal, thromboembolic events causing myocardial infarction and stroke can occur during angiocardiography procedures with both ionic and nonionic contrast media. During these procedures, increased thrombosis and activation of the complement system occurs. Risk factors for thromboembolic events include: length of procedure, catheter and syringe material, underlying disease state, and concomitant medications.
To minimize thromboembolic events, use meticulous angiographic techniques, and minimize the length of the procedure. Avoid blood remaining in contact with syringes containing iodinated contrast agents, which increases the risk of clotting. Avoid angiocardiography in patients with homocystinuria because of the risk of inducing thrombosis and embolism.
5.7 Extravasation and Injection Site Reactions
Extravasation of OMNIPAQUE during intravascular injection may cause tissue necrosis and/or compartment syndrome, particularly in patients with severe arterial or venous disease. Ensure intravascular placement of catheters prior to injection. Monitor patients for extravasation and advise patients to seek medical care for progression of symptoms.
5.8 Thyroid Storm in Patients with Hyperthyroidism
Thyroid storm has occurred after the intravascular use of iodinated contrast agents in patients with hyperthyroidism, or with an autonomously functioning thyroid nodule. Evaluate the risk in such patients before use of OMNIPAQUE.
5.9 Hypertensive Crisis in Patients with Pheochromocytoma
Hypertensive crisis has occurred after the use of iodinated contrast agents in patient with pheochromocytoma. Monitor patients when administering OMNIPAQUE intravascularly if pheochromocytoma or catecholamine-secreting paragangliomas are suspected. Inject the minimum amount of contrast necessary, assess the blood pressure throughout the procedure, and have measures for treatment of a hypertensive crisis readily available.
5.10 Sickle Cell Crisis in Patients with Sickle Cell Disease
Iodinated contrast agents when administered intravascularly may promote sickling in individuals who are homozygous for sickle cell disease. Hydrate patients prior to and following OMNIPAQUE administration and use OMNIPAQUE only if the necessary imaging information cannot be obtained with alternative imaging modalities.
5.11 Severe Cutaneous Adverse Reactions
Severe cutaneous adverse reactions (SCAR) may develop from 1 hour to several weeks after intravascular contrast agent administration. These reactions include Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN), acute generalized exanthematous pustulosis (AGEP) and drug reaction with eosinophilia and systemic symptoms (DRESS). Reaction severity may increase and time to onset may decrease with repeat administration of contrast agents; prophylactic medications may not prevent or mitigate severe cutaneous adverse reactions. Avoid administering OMNIPAQUE to patients with a history of a severe cutaneous adverse reaction to OMNIPAQUE.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Risks Associated with Inadvertent Intrathecal Administration [see Warnings and Precautions (5.1)]
- Risks Associated with Inadvertent Parenteral Administration [see Warnings and Precautions (5.2)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.3)]
- Contrast Induced Kidney Injury [see Warnings and Precautions (5.4)]
- Cardiovascular Adverse Reactions [see Warnings and Precautions (5.5)]
- Thromboembolic Events [see Warnings and Precautions (5.6)]
- Severe Cutaneous Reactions [see Warnings and Precautions (5.11)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Intrathecal Administration
Adults
TABLE 23 ADVERSE REACTIONS – INTRATHECAL ADMINISTRATION In controlled clinical studies involving 1531 patients using OMNIPAQUE the following adverse reactions were reported: System Organ Class Adverse Reaction Incidence Nervous System Headaches 18% Musculoskeletal and Connective Tissue Pain including backache, neckache, stiffness and neuralgia 8% Gastrointestinal System Nausea 6% Vomiting 3% Nervous System Dizziness 2% Other Reactions Feeling of heaviness, hypotension, hypertonia, sensation of heat, sweating, vertigo, loss of appetite, drowsiness, hypertension, photophobia, tinnitus, neuralgia, paresthesia, difficulty in micturition, and neurological changes <0.1% Pediatric Patients
TABLE 24 ADVERSE REACTIONS – INTRATHECAL ADMINISTRATION In clinical studies involving 152 patients for pediatric myelography by lumbar puncture, adverse events following the use of OMNIPAQUE 180 were generally similar to those reported in adults. Procedure System Organ Class Adverse Reaction Incidence Myelography by Lumbar Puncture Nervous System Headache 9% Gastrointestinal System Vomiting 6% Musculoskeletal and Connective Tissue Backache 1.3% Other Reactions
All were transient and mild with no clinical sequelae.Fever <0.7% Hives Stomachache Visual Hallucination Neurological Changes Intravascular Administration
Immediately following intravascular injection of contrast medium, a transient sensation of mild warmth is not unusual. Warmth is less frequent with OMNIPAQUE than with ionic contrast media.
Adults
In controlled clinical studies involving 1485 patients, the following adverse reactions occurred (Table 25):
TABLE 25 ADVERSE REACTIONS – INTRAVASCULAR ADMINISTRATION System Organ Class Adverse Reaction Incidence Cardiovascular System Arrhythmias including PVCs and PACs (2%), 2% Hypotension 0.7% Others including cardiac failure, asystole, bradycardia, tachycardia, and vasovagal reaction ≤ 0.3% Nervous System Vertigo (including dizziness and lightheadedness) 0.5% Pain 3% Vision Abnormalities (including blurred vision and photomas) 2% Taste Perversion 1% Other Reactions Anxiety, fever, motor and speech dysfunction, convulsion, paresthesia, somnolence, stiff neck, hemiparesis, syncope, shivering, transient ischemic attack, cerebral infarction, and nystagmus Individual incidence of 0.3% or less Respiratory System Dyspnea, rhinitis, coughing, and laryngitis Individual incidence of 0.2% or less Gastrointestinal System Nausea 2% Vomiting 0.7% Others including diarrhea, dyspepsia, cramp, and dry mouth Individual incidence of less than 0.1%. Skin and Subcutaneous Tissues Urticaria 0.3% Purpura 0.1% Abscess 0.1% Pruritus 0.1% Pediatric Patients
In controlled clinical studies involving 391 patients for pediatric angiocardiography, urography, and CT head imaging, adverse reactions following the use of OMNIPAQUE 240, 300, and 350 were generally similar in quality and frequency to those reported in adults (Table 26):
TABLE 26 ADVERSE REACTIONS – INTRAVASCULAR ADMINISTRATION System Organ Class Adverse Reaction Incidence Cardiovascular System Ventricular Tachycardia 0.5% 2:1 Heart Block 0.5% Hypertension 0.3% Anemia 0.3% General Disorders and Administration Site Conditions Pain 0.8% Fever 0.5% Nervous System Convulsion 0.3% Taste Abnormality 0.5% Respiratory System Congestion 0.3% Apnea 0.3% Gastrointestinal System Nausea 1% Vomiting 2% Endocrine System Hypoglycemia 0.3% Skin and Subcutaneous Tissue Rash 0.3% Oral Administration for Examination of the Gastrointestinal Tract
Adults
Nausea, vomiting, and diarrhea have been most frequently reported following orally administered undiluted OMNIPAQUE for radiographic examination of the gastrointestinal tract. In controlled clinical studies involving 54 adult patients for oral radiographic examination of the gastrointestinal tract using undiluted OMNIPAQUE 350 the following adverse reactions were reported (Table 27):
TABLE 27 ADVERSE REACTIONS – ORAL ADMINISTRATION OF UNDILUTED OMNIPAQUE 350 System Organ Class Adverse Reaction Incidence Gastrointestinal System Diarrhea 42% Nausea 15% Vomiting 11% Abdominal Pain 7% Flatulence 2% Nervous System Headache 2% Pediatrics Patients (Oral and Rectal Administration)
In clinical studies involving 58 pediatric patients, the adverse reactions were found to mostly affect the gastrointestinal system with diarrhea (36%), vomiting (9%), nausea (5%) and abdominal pain (2%). However, fever (5%), hypotension (2%) and urticaria (2%) were also reported.
Oral Administration for CT of the Abdomen in Conjunction with Intravenous Administration
Adults
In a controlled clinical study involving 44 adult patients receiving oral administration of diluted OMNIPAQUE (4-9 mg iodine/mL) in conjunction with intravenously injected OMNIPAQUE 300 for CT examination of the abdomen, adverse reactions were limited to a single report of vomiting.
Pediatric Patients
In clinical studies involving 69 pediatric patients receiving oral administration of diluted OMNIPAQUE (9-29 mg iodine/mL) in conjunction with intravenously administered OMNIPAQUE 240 and OMNIPAQUE 300 for CT examination of the abdomen, adverse reactions were limited to a single report of vomiting (1.4%).
Body Cavity Use
Adults
In controlled clinical studies involving 285 adult patients for various body cavity examinations using OMNIPAQUE 240, 300 and 350, the most frequent adverse reactions were administration site reactions: pain 26% and swelling 22%, were exclusively reported for arthrography and were generally related to the procedure rather than the contrast medium. Patients also experienced heat (7%). All other adverse reaction occurred at a rate less than or equal to 1%.
6.2 Post-marketing Experience
The following additional reactions listed by indication have been identified during post-approval use of OMNIPAQUE. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
General
Immune System Disorders: Hypersensitivity reactions, anaphylactic or anaphylactoid reactions, anaphylactic or anaphylactoid shock including life-threatening or fatal anaphylaxis.
General Disorders and Administration Site Conditions: Pyrexia, chills, pain and discomfort, asthenia, administration site conditions including extravasation.
Intrathecal Administration
Nervous System Disorders: Meningism, aseptic meningitis, seizures or status epilepticus, disorientation, coma, depressed or loss of consciousness, transient contrast-induced toxic encephalopathy (including amnesia, hallucination, paralysis, paresis, speech disorder, aphasia, dysarthria), restlessness, tremors, hypoesthesia.
Musculoskeletal and Connective Tissue Disorders: Pain, muscle spasms or spasticity.
Psychiatric Disorders: Confusional state, agitation, anxiety.
Eye Disorders: Transient visual impairment including cortical blindness.
Renal Reactions: Acute kidney injury.
Intravascular Administration
Cardiovascular Disorders: Severe cardiac complications (including cardiac arrest, cardiopulmonary arrest), shock, peripheral vasodilatation, palpitations, vasospasm including spasm of coronary arteries, myocardial infarction, syncope, cyanosis, pallor, flushing, chest pain.
Hemodynamic Reactions: Vasospasm and thrombophlebitis following intravenous injection.
Blood and Lymphatic System Disorders: Neutropenia.
Nervous System Disorders: Disorientation, coma, depressed or loss of consciousness, transient contrast-induced toxic encephalopathy (including amnesia, hallucination, paralysis, paresis, speech disorder, aphasia, dysarthria), restlessness, tremors, hypoesthesia.
Psychiatric Disorders: Confusional state, agitation.
Eye Disorders: Eye irritation or itchiness, periorbital edema, ocular or conjunctival hyperemia, lacrimation.
Renal Reactions: Acute kidney injury, toxic nephropathy (CIN), transient proteinuria, oliguria or anuria, increased serum creatinine.
Gastrointestinal Disorders: Abdominal pain, pancreatitis aggravated, salivary gland enlargement.
Endocrine Reactions: Hyperthyroidism. Thyroid function tests indicative of hypothyroidism or transient thyroid suppression have been uncommonly reported following iodinated contrast media administration to adult and pediatric patients, including infants. Some patients were treated for hypothyroidism.
Respiratory; Thoracic, and Mediastinal Disorders: Respiratory distress, respiratory failure, pulmonary edema, bronchospasm, laryngospasm, throat irritation, throat tightness, laryngeal edema, wheezing, chest discomfort, asthmatic attack.
Skin and Subcutaneous Tissue Disorders: Contrast media reactions range from mild (e.g. pleomorphic rashes, drug eruption, erythema and skin discoloration, blisters, hyperhidrosis, angioedema, localized areas of edema) to severe: [e.g. Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN), bullous or exfoliative dermatitis, acute generalized exanthematous pustulosis (AGEP) and drug reaction with eosinophilia and systemic symptoms (DRESS)].
-
7 DRUG INTERACTIONS
7.1 Drug-Drug Interactions
Metformin
In patients with renal impairment, metformin can cause lactic acidosis. Iodinated contrast agents appear to increase the risk of metformin-induced lactic acidosis, possibly as a result of worsening renal function. Stop metformin at the time of, or prior to, OMNIPAQUE administration in patients with an eGFR between 30 and 60 mL/min/1.73 m2; in patients with a history of hepatic impairment, alcoholism or heart failure; or in patients who will be administered intra-arterial iodinated contrast. Re-evaluate eGFR 48 hours after the imaging procedure, and reinstitute metformin only after renal function is stable.
Radioactive Iodine
Administration of iodinated contrast agents may interfere with thyroid uptake of radioactive iodine (I-131 and I-123) and decrease therapeutic and diagnostic efficacy in patients with carcinoma of the thyroid. The decrease in efficacy lasts for 6-8 weeks.
Beta-adrenergic Blocking Agents
The use of beta-adrenergic blocking agents lowers the threshold for and increases the severity of contrast reactions and reduces the responsiveness of treatment of hypersensitivity reactions with epinephrine. Because of the risk of hypersensitivity reactions, use caution when administering OMNIPAQUE to patients taking beta-blockers.
Drugs that Lower Seizure Threshold
Drugs that lower seizure threshold, especially phenothiazine derivatives including those used for their antihistaminic or antinauseant properties, are not recommended for use with intrathecal administration of OMNIPAQUE.
CNS Active Drugs
Drugs such as monoamine oxidase (MAO) inhibitors, tricyclic antidepressants, CNS stimulants, psychoactive drugs described as analeptics, major tranquilizers, or antipsychotic drugs. Such medications should be discontinued at least 48 hours before myelography, should not be used for the control of nausea or vomiting during or after myelography, and should not be resumed for at least 24 hours post procedure. In non-elective procedures in patients on these drugs, consider prophylactic use of anticonvulsants.
7.2 Drug Laboratory Test Interactions
Effect on Thyroid Tests
If iodine-containing isotopes are to be administered for the diagnosis of thyroid disease, the iodine-binding capacity of thyroid tissue may be reduced for up to 2 weeks after contrast medium administration. Thyroid function tests that do not depend on iodine estimation, e.g., T3 resin uptake or direct thyroxine assays, are not affected.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no data with iohexol use in pregnant women to inform any drug-associated risks. Iohexol crosses the placenta and reaches fetal tissues in small amounts (see Data). In animal reproduction studies, no developmental toxicity occurred with intravenous iohexol administration to rats and rabbits at doses up to 0.4 (rat) and 0.5 (rabbit) times the maximum recommended human intravenous dose (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Human Data
Literature reports show that intravenously administered iohexol crosses the placenta and is visualized in the digestive tract of exposed infants after birth.
Animal Data
Iohexol was neither embryotoxic nor teratogenic in either rats or rabbits at the following dose levels tested: 1.0, 2.0, 4.0 g iodine/kg in rats, administered intravenously to 3 groups of 25 dams once daily during days 6 through 15 of pregnancy; 0.3, 1.0, 2.5 g iodine/kg in rabbits, administered intravenously to 3 groups of 18 rabbits dosed once a day during days 6 through 18 of pregnancy.
8.2 Lactation
Risk Summary
Published literature reports that breast feeding after intravenous iohexol administration to the mother would result in the infant receiving an oral dose of approximately 0.7% of the maternal intravenous dose; however, lactation studies have not been conducted with oral, intrathecal, or intracavity administration of iohexol. There is no information on the effects of the drug on the breastfed infant or on milk production. Iodinated contrast agents are excreted unchanged in human milk in very low amounts with poor absorption from the gastrointestinal tract of a breastfed infant. Exposure to iohexol to a breastfed infant can be minimized by temporary discontinuation of breastfeeding (see Clinical Considerations). The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for OMNIPAQUE and any potential adverse effects on the breastfed infant from OMNIPAQUE or from the underlying maternal condition.
Clinical Considerations
Interruption of breastfeeding after exposure to iodinated contrast agents is not necessary because the potential exposure of the breastfed infant to iodine is small. However, a lactating woman may consider interrupting breastfeeding and pumping and discarding breast milk for 10 hours (approximately 5 elimination half-lives) after OMNIPAQUE administration to minimize drug exposure to a breastfed infant.
8.4 Pediatric Use
Intrathecal Use
The safety and effectiveness of OMNIPAQUE 180 have been established in pediatric patients 2 weeks to 17 years of age for myelography (lumbar, thoracic, cervical, total columnar) and for CT (myelography, cisternography). Use of OMNIPAQUE 180 is supported by controlled clinical studies in adults for myelography, in addition to clinical studies in pediatric patients undergoing myelography. The safety and effectiveness of OMNIPAQUE 180 have not been established for intrathecal use in patient pediatric patients less than 2 weeks of age. The safety and effectiveness of OMNIPAQUE 240 and 300 have not been established in pediatric patients for myelography (lumbar, thoracic, cervical, total columnar) and for CT (myelography, cisternography, or ventriculography).
Intravascular Use
Angiocardiography (Ventriculography, Pulmonary Arteriography, Venography, and Studies of the Collateral Arteries) and Aortography
The safety and effectiveness of OMNIPAQUE 300 have been established in pediatric patients from birth to 17 years of age for angiocardiography (ventriculography) and of OMNIPAQUE 350 in pediatric patients from birth to 17 years of age for angiocardiography (ventriculography, pulmonary arteriography, venography, and studies of the collateral arteries) and aortography. Use of OMNIPAQUE 300 and 350 is supported by controlled clinical studies in adults for angiocardiography and aortography, in addition to controlled clinical studies in pediatric patients undergoing angiocardiography, including aortography. The safety and effectiveness of OMNIPAQUE 300 have not been established in pediatric patients for aortography.
Intra-arterial Digital Subtraction Angiography, Intravenous Digital Subtraction Angiography, Cerebral Arteriography, or Peripheral Arteriography and Venography
The safety and effectiveness of OMNIPAQUE have not been established in pediatric patients for intra-arterial digital subtraction angiography, intravenous digital subtraction angiography, cerebral arteriography, or peripheral arteriography and venography.
CT of the Head and Body
The safety and effectiveness of OMNIPAQUE 240 and 300 have been established in pediatric patients from birth to 17 years of age for CT imaging of the head and body. Use of OMNIPAQUE 240 and 300 is supported by controlled clinical studies in adults for head and body CT, in addition to clinical studies in pediatric patients undergoing head CT and in 69 pediatric patients undergoing CT of the abdomen after oral administration of diluted OMNIPAQUE plus intravenous administration of OMNIPAQUE. The safety and effectiveness of OMNIPAQUE 350 have not been established in pediatric patients for CT imaging of the head and body.
Urography
The safety and effectiveness of OMNIPQUE 300 have been established in pediatric patients from birth to 17 years of age for urography. Use of OMNIPAQUE 300 is supported by controlled clinical studies in adults for urography, in addition to controlled clinical studies in pediatric patients undergoing urography and clinical safety data in pediatric patients down to birth.
Oral or Rectal Use.
Undiluted OMNIPAQUE Injection
The safety and effectiveness of OMNIPAQUE 180, 240, and 300 administered orally and rectally have been established in pediatric patients, from birth to 17 years of age for examination of the GI tract. Use of OMNIPAQUE 180, 240, and 300 administered orally and rectally is supported by controlled studies in adults for examination of the GI tract, in addition to clinical studies in pediatric patients undergoing examination of the GI tract.
Oral Use in Conjunction with Intravenous Use
Diluted OMNIPAQUE Injection
The safety and effectiveness of OMNIPAQUE injection diluted to concentrations from 9 to 21 mg iodine/mL administered orally in conjunction with OMNIPAQUE injection administered intravenously for CT of the abdomen have been established in pediatric patients from birth to 17 years of age. Use is supported by clinical trials in adults, in addition to clinical studies in 69 pediatric patients undergoing CT of the abdomen after oral administration of diluted OMNIPAQUE plus intravenous administration of OMNIPAQUE.
OMNIPAQUE Oral Solution
The safety and effectiveness of OMNIPAQUE oral solution 9 and 12 administered orally in conjunction with OMNIPAQUE injection administered intravenously for CT of the abdomen in pediatric patients have been established in pediatric patients from birth to 17 years of age. Use is supported by the data establishing safety and effectiveness for OMNIPAQUE injection diluted and administered orally in conjunction with OMNIPAQUE injection administered intravenously for CT of the abdomen in pediatric patients.
Intraarticular Use
The safety and effectiveness of OMNIPAQUE have not been established in pediatric patients for arthrography.
Body Cavity Use
OMNIPAQUE 240, 300, 350 diluted to concentrations from 50 mg iodine/mL to 100 mg iodine/mL is indicated for use in pediatric patients from birth to 17 years of age for voiding cystourethrography (VCU). The use for voiding cystourethrography is supported by clinical studies in 51 pediatric patients undergoing VCU. The safety and effectiveness of OMNIPAQUE have not been established in pediatric patients for ERCP, herniography, or hysterosalpingography.
In general, the frequency of adverse reactions in pediatric patients was similar to that seen in adults [see Adverse Reactions (6.1)]. Pediatric patients at higher risk of experiencing adverse events during contrast-medium administration may include those having asthma, a sensitivity to medication and/or allergens, congestive heart failure, a serum creatinine greater than 1.5 mg/dL or those less than 12 months of age.
Thyroid function tests indicative of hypothyroidism or transient thyroid suppression have been uncommonly reported following iodinated contrast media administration to pediatric patients, including infants. Some patients were treated for hypothyroidism [see Adverse Reactions (6.2)].
8.5 Geriatric Use
In clinical studies of OMNIPAQUE for CT, 52/299 (17%) of patients were 70 and over. No overall differences in safety were observed between these patients and younger patients. Other reported clinical experience has not identified differences in response between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out. In general, dose selection for an elderly patient should be cautious usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
-
10 OVERDOSAGE
10.1 Intravascular Administration
The adverse effects of overdosage are life-threatening and affect mainly the pulmonary and cardiovascular systems. The symptoms included: cyanosis, bradycardia, acidosis, pulmonary hemorrhage, convulsions, coma, and cardiac arrest. Treatment of an overdosage is directed toward the support of all vital functions, and prompt institution of symptomatic therapy. Iohexol displays a low affinity for serum or plasma proteins and is poorly bound to serum albumin and can be dialyzed.
-
11 DESCRIPTION
11.1 Chemical Characteristics
OMNIPAQUE (iohexol) injection is a nonionic, x-ray or radiographic contrast medium for intrathecal, intravenous, oral, rectal and body cavity use. OMNIPAQUE oral solution is for oral use only.
OMNIPAQUE injection and OMNIPAQUE oral solution are both provided as sterile, pyrogen-free and gluten-free solutions. OMNIPAQUE injection and OMNIPAQUE oral solution are colorless to pale yellow solutions. The chemical name of iohexol is Bis(2,3-dihydroxypropyl)-5-[N-(2,3-dihydroxypropyl)-acetamido]-2,4,6- triiodoisophthalamide with a molecular weight of 821.14 (iodine content 46.36%). Iohexol has the following structural formula:
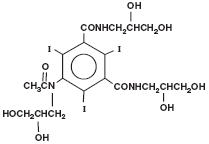
OMNIPAQUE injection is available in five strengths:
- OMNIPAQUE 140 mg iodine/mL (302 mg of iohexol/mL): Each mL contains 140 mg organically bound iodine, 1.21 mg tromethamine and 0.1 mg edetate calcium disodium
- OMNIPAQUE 180 mg iodine/mL (388 mg of iohexol/mL): Each mL contains 180 mg organically bound iodine, 1.21 mg tromethamine and 0.1 mg edetate calcium disodium
- OMNIPAQUE 240 mg iodine/mL (518 mg of iohexol/mL): Each mL contains 240 mg organically bound iodine, 1.21 mg tromethamine and 0.1 mg edetate calcium disodium
- OMNIPAQUE 300 mg iodine/mL (647 mg of iohexol/mL): Each mL contains 300 mg organically bound iodine, 1.21 mg tromethamine and 0.1 mg edetate calcium disodium
- OMNIPAQUE 350 mg iodine/mL (755 mg of iohexol/mL): Each mL contains 350 mg organically bound iodine, 1.21 mg tromethamine and 0.1 mg edetate calcium disodium
OMNIPAQUE oral solution is available in two strengths:
- OMNIPAQUE oral solution 9 mg iodine/mL (19 mg of iohexol/mL): Each mL contains 9 mg organically bound iodine, 1.21 mg tromethamine and 0.1 mg edetate calcium disodium
- OMNIPAQUE oral solution 12 mg iodine/mL (26 mg of iohexol/mL): Each mL contains 12 mg organically bound iodine, 1.21 mg tromethamine and 0.1 mg edetate calcium disodium
The pH is adjusted between 6.8 and 7.7 with hydrochloric acid or sodium hydroxide. OMNIPAQUE injection and OMNIPAQUE oral solution are sterilized by autoclaving and contain no preservatives.
11.2 Physical Characteristics
OMNIPAQUE injection and OMNIPAQUE oral solution have the following physical properties:
Presentation Concentration
(mg iodine/mL)Osmolality*
(mOsmol/kg water)Absolute Viscosity
(cP)Specific Gravity 20°C 37°C 37°C - * By vapor-pressure osmometry.
OMNIPAQUE 140 140 322 2.3 1.5 1.164 OMNIPAQUE 180 180 408 3.1 2.0 1.209 OMNIPAQUE 240 240 520 5.8 3.4 1.280 OMNIPAQUE 300 300 672 11.8 6.3 1.349 OMNIPAQUE 350 350 844 20.4 10.4 1.406 OMNIPAQUE oral solution 9 9 38 1.1 0.8 1.011 OMNIPAQUE oral solution 12 12 45 1.1 0.8 1.014 OMNIPAQUE 140, OMNIPAQUE 180, OMNIPAQUE 240, OMNIPAQUE 300, and OMNIPAQUE 350 have osmolalities from approximately 1.1 to 3.0 times that of plasma (285 mOsmol/kg water) or cerebrospinal fluid (301 mOsmol/kg water) as shown in the above table and are hypertonic under conditions of use.
OMNIPAQUE oral solution 9 and OMNIPAQUE oral solution 12 are hypotonic under conditions of use (see table above).
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The iodine atoms in iohexol provide attenuation of X-rays in direct proportion to the concentration of iohexol. Since concentration changes over time, iohexol provides time-dependent image contrast which may assist in visualizing body structures.
12.2 Pharmacodynamics
Intrathecal Administration
The initial concentration and volume of the contrast medium, in conjunction with patient manipulation and the volume of cerebrospinal fluid (CSF) into which the contrast medium is placed, will determine the extent of the contrast that can be achieved. Following intrathecal injection in conventional radiography, OMNIPAQUE 180, 240, and 300 will continue to provide contrast for at least 30 minutes. Slow diffusion of iohexol takes place throughout the CSF with subsequent absorption into the bloodstream. At approximately 1 hour following injection, contrast will no longer be sufficient for conventional myelography.
After administration into the lumbar subarachnoid space, computerized tomography shows the presence of contrast medium in the thoracic region in about 1 hour, in the cervical region in about 2 hours, and in the basal cisterns in 3 to 4 hours.
Intravascular Administration
Following intravascular administration of OMNIPAQUE, the degree of contrast enhancement is directly related to the iodine concentration of an administered dose; peak iodine blood concentrations occur immediately (15 seconds to 120 seconds) following rapid intravenous injection. The time to maximum contrast enhancement can vary, depending on the organ, from the time that peak blood iodine concentrations are reached to one hour after intravenous bolus administration. When a delay between peak blood iodine concentrations and peak contrast is present, it suggests that radiographic contrast enhancement is at least in part dependent on the accumulation of iodine containing agent within the lesion and outside the blood pool.
Oral Administration
Orally administered OMNIPAQUE produces visualization of the gastrointestinal tract. Less than 1% of orally administered iohexol is recovered in the urine, suggesting minimal amounts are absorbed from the normal gastrointestinal tract. This amount may increase in the presence of bowel perforation or bowel obstruction.
Intraarticular Administration
Visualization of the joint spaces can be accomplished by direct injection of contrast medium. For intraarticular cavities, the injected iohexol is absorbed into the surrounding tissue and subsequently absorbed into systemic circulation.
Body Cavity Administration
For most body cavities, the injected iohexol is absorbed into the surrounding tissue and subsequently absorbed into systemic circulation. Examinations of the uterus (hysterosalpingography) and bladder (voiding cystourethrography) involve the almost immediate drainage of contrast medium from the cavity upon conclusion of the radiographic procedure.
12.3 Pharmacokinetics
Following the intravenous administration of iohexol (between 500 mg iodine/kg to 1500 mg iodine/kg) to 16 adult human subjects, apparent first-order terminal elimination half-life was 12.6 hrs and total body clearance was 131 (98-165) mL/min. Clearance was not dose dependent.
Absorption
As evidenced by the amount recovered in urine, <1% of orally administered iohexol is absorbed from the normal gastrointestinal tract. This amount may increase in the presence of bowel perforation or bowel obstruction.
Distribution
In 16 adult subjects (receiving between 500 mg iodine/kg to 1500 mg iodine/kg intravenous iohexol) the plasma volume of distribution was165 (108-219) mL/kg.
In five adult patients receiving 16 mL to 18 mL of OMNIPAQUE (180 mg iodine/mL) by lumbar intrathecal injection the plasma volume of distribution was 559 (350-849) mL/kg.
Elimination
Excretion
Following intravascular or intrathecal administration, iohexol is excreted unchanged by glomerular filtration. Approximately 90% of the intravenously injected iohexol dose is excreted within the first 24 hours. Following intravascular administration, peak urine concentration occurs in the first hour after injection.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies have not been performed with iohexol to evaluate carcinogenic potential. Iohexol was not genotoxic in a series of studies, including the Ames test, the mouse lymphoma TK locus forward mutation assay, and a mouse micronucleus assay. Iohexol did not impair the fertility of male or female rats when repeatedly administered at intravenous dosages up to 4 g iodine/kg.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Volume/Concentration Configuration NDC OMNIPAQUE 240 (240 mg iodine/mL) – Boxes of 10 50 mL +PLUSPAK™ (polymer bottle) 0407-1412-38 100 mL fill in 100 mL bottle with hanger 0407-1412-84 100 mL +PLUSPAK™ (polymer bottle) 0407-1412-39 150 mL +PLUSPAK™ (polymer bottle) 0407-1412-40 OMNIPAQUE 300 (300 mg iodine/mL) – Boxes of 10 50 mL Glass Vial 0407-1413-41 50 mL bottle with hanger 0407-1413-42 50 mL +PLUSPAK™ (polymer bottle) 0407-1413-86 100 mL fill in 100 mL bottle with hanger 0407-1413-43 100 mL +PLUSPAK™ (polymer bottle) 0407-1413-87 125 mL in 200 mL bottle with hanger 0407-1413-47 150 mL fill in 200 mL bottle with hanger 0407-1413-46 150 mL +PLUSPAK™ (polymer bottle) 0407-1413-88 OMNIPAQUE 350 (350 mg iodine/mL) – Boxes of 10 50 mL Glass Vial 0407-1414-43 50 mL bottle with hanger 0407-1414-44 50 mL +PLUSPAK™ (polymer bottle), 0407-1414-82 100 mL fill in 100 mL bottle with hanger 0407-1414-45 100 mL +PLUSPAK™ (polymer bottle) 0407-1414-84 125 mL fill in 200 mL bottle with hanger 0407-1414-85 150 mL fill in 200 mL bottle with hanger 0407-1414-41 150 mL +PLUSPAK™ (polymer bottle) 0407-1414-86 200 mL fill in 200 mL bottle with hanger 0407-1414-42 200 mL +PLUSPAK™ (polymer bottle) 0407-1414-87 The container closure system components (bottle, vial, stopper and cap) of OMNIPAQUE injection and OMNIPAQUE oral solution are not made with natural rubber latex.
16.2 Storage
Protect OMNIPAQUE glass vials and bottles and +PLUSPAK™ polymer bottles from light. Do not freeze. Discard any product that is inadvertently frozen, as freezing may compromise the closure integrity of the immediate container.
-
17 PATIENT COUNSELING INFORMATION
Hypersensitivity Reactions
Advise the patient concerning the risk of hypersensitivity reactions that can occur both during and after OMNIPAQUE administration. Advise the patient to report any signs or symptoms of hypersensitivity reactions during the procedure and to seek immediate medical attention for any signs or symptoms experienced after discharge [see Warnings and Precautions (5.3)]
Advise patients to inform their physician if they develop a rash after receiving OMNIPAQUE [see Warnings and Precautions (5.11)].
Contrast Induced Acute Kidney Injury
Advise the patient concerning appropriate hydration to decrease the risk of contrast induced acute kidney injury [see Warnings and Precautions (5.4)].
Extravasation
If extravasation occurs during injection, advise patients to seek medical care for progression of symptoms [see Warnings and Precautions (5.7)].
Lactation
Advise a lactating woman that interruption of breastfeeding is not necessary. However, to avoid any exposure, a lactating woman may consider pumping and discarding breast milk for 10 hours after OMNIPAQUE administration [see Use in Specific Populations (8.2)].
-
SPL UNCLASSIFIED SECTION
NOVAPLUS ®
Distributed by GE Healthcare Inc., Marlborough, MA 01752 U.S.A.
Manufactured by GE Healthcare Ireland Limited, Cork, IrelandGE Healthcare
OMNIPAQUE is a trademark of General Electric Company or one of its subsidiaries.
GE and the GE Monogram are trademarks of General Electric Company.
NOVAPLUS is a registered trademark of Vizient, Inc.
Product of Norwegian origin.© 2018 General Electric Company - All rights reserved.
OVH-3M-CORK
Revised April 2018 -
PRINCIPAL DISPLAY PANEL - 50 mL Bottle Box Label - 1412
520-Y
Contains
10 x 50 mL
BottlesNDC: 0407-1412-38
Omnipaque™
(iohexol)
Injection240
mgI/mL50 mL
NOVAPLUS®
Single-Dose Bottle.
Sterile Aqueous Solution
For Injection or Oral Use.
-
PRINCIPAL DISPLAY PANEL - 50 mL Bottle Box Label - 1413
530-Y
Contains
10 x 50 mL
BottlesNDC: 0407-1413-86
Omnipaque™
(iohexol)
Injection300
mgI/mL50 mL
NOVAPLUS®
Single-Dose Bottle.
Sterile Aqueous Solution
For Injection or Oral Use.
-
PRINCIPAL DISPLAY PANEL - 50 mL Bottle Box Label - 1414
540-Y
Contains
10 x 50 mL
BottlesNDC: 0407-1414-82
Omnipaque™
(iohexol)
InjectionNOT FOR
INTRATHECAL
USE350
mgI/mL50 mL
NOVAPLUS®
Single-Dose Bottle.
Sterile Aqueous Solution
For Injection or Oral Use.
-
INGREDIENTS AND APPEARANCE
OMNIPAQUE
iohexol injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0407-1412 Route of Administration INTRATHECAL, INTRAVASCULAR, INTRAVENOUS, ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Iohexol (UNII: 4419T9MX03) (Iohexol - UNII:4419T9MX03) Iodine 240 mg in 1 mL Inactive Ingredients Ingredient Name Strength Iodine (UNII: 9679TC07X4) 240 mg in 1 mL tromethamine (UNII: 023C2WHX2V) 1.21 mg in 1 mL edetate calcium disodium (UNII: 25IH6R4SGF) 0.1 mg in 1 mL hydrochloric acid (UNII: QTT17582CB) sodium hydroxide (UNII: 55X04QC32I) Product Characteristics Color YELLOW (colorless to pale yellow) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0407-1412-38 10 in 1 BOX 08/11/2005 1 50 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 2 NDC: 0407-1412-84 10 in 1 BOX 08/11/2005 2 100 mL in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC: 0407-1412-39 10 in 1 BOX 08/11/2005 3 100 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 4 NDC: 0407-1412-40 10 in 1 BOX 08/11/2005 4 150 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA018956 08/11/2005 OMNIPAQUE
iohexol injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0407-1413 Route of Administration INTRATHECAL, INTRAVASCULAR, INTRAVENOUS, ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Iohexol (UNII: 4419T9MX03) (Iohexol - UNII:4419T9MX03) Iodine 300 mg in 1 mL Inactive Ingredients Ingredient Name Strength Iodine (UNII: 9679TC07X4) 300 mg in 1 mL tromethamine (UNII: 023C2WHX2V) 1.21 mg in 1 mL edetate calcium disodium (UNII: 25IH6R4SGF) 0.1 mg in 1 mL hydrochloric acid (UNII: QTT17582CB) sodium hydroxide (UNII: 55X04QC32I) Product Characteristics Color YELLOW (colorless to pale yellow) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0407-1413-41 10 in 1 BOX 09/21/2006 1 50 mL in 1 VIAL; Type 0: Not a Combination Product 2 NDC: 0407-1413-42 10 in 1 BOX 09/21/2006 2 50 mL in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC: 0407-1413-43 10 in 1 BOX 09/21/2006 3 100 mL in 1 BOTTLE; Type 0: Not a Combination Product 4 NDC: 0407-1413-86 10 in 1 BOX 09/21/2006 4 50 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 5 NDC: 0407-1413-87 10 in 1 BOX 09/21/2006 5 100 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 6 NDC: 0407-1413-47 10 in 1 BOX 09/21/2006 6 125 mL in 1 BOTTLE; Type 0: Not a Combination Product 7 NDC: 0407-1413-46 10 in 1 BOX 09/21/2006 7 150 mL in 1 BOTTLE; Type 0: Not a Combination Product 8 NDC: 0407-1413-88 10 in 1 BOX 09/21/2006 8 150 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA018956 09/21/2006 OMNIPAQUE
iohexol injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0407-1414 Route of Administration INTRAVASCULAR, INTRAVENOUS, ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Iohexol (UNII: 4419T9MX03) (Iohexol - UNII:4419T9MX03) Iodine 350 mg in 1 mL Inactive Ingredients Ingredient Name Strength Iodine (UNII: 9679TC07X4) 350 mg in 1 mL tromethamine (UNII: 023C2WHX2V) 1.21 mg in 1 mL edetate calcium disodium (UNII: 25IH6R4SGF) 0.1 mg in 1 mL hydrochloric acid (UNII: QTT17582CB) sodium hydroxide (UNII: 55X04QC32I) Product Characteristics Color YELLOW (colorless to pale yellow) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0407-1414-43 10 in 1 BOX 07/21/2006 1 50 mL in 1 VIAL; Type 0: Not a Combination Product 2 NDC: 0407-1414-44 10 in 1 BOX 07/21/2006 2 50 mL in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC: 0407-1414-82 10 in 1 BOX 07/21/2006 3 50 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 4 NDC: 0407-1414-45 10 in 1 BOX 07/21/2006 4 100 mL in 1 BOTTLE; Type 0: Not a Combination Product 5 NDC: 0407-1414-84 10 in 1 BOX 07/21/2006 5 100 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 6 NDC: 0407-1414-85 10 in 1 BOX 07/21/2006 6 125 mL in 1 BOTTLE; Type 0: Not a Combination Product 7 NDC: 0407-1414-41 10 in 1 BOX 07/21/2006 7 150 mL in 1 BOTTLE; Type 0: Not a Combination Product 8 NDC: 0407-1414-86 10 in 1 BOX 07/21/2006 8 150 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 9 NDC: 0407-1414-42 10 in 1 BOX 07/21/2006 9 200 mL in 1 BOTTLE; Type 0: Not a Combination Product 10 NDC: 0407-1414-87 10 in 1 BOX 07/21/2006 10 200 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA018956 07/21/2006 Labeler - GE Healthcare Inc. (053046579) Establishment Name Address ID/FEI Business Operations GE Healthcare Shanghai, Co., Ltd. 545292716 MANUFACTURE(0407-1412, 0407-1413, 0407-1414) , REPACK(0407-1412, 0407-1413, 0407-1414) , RELABEL(0407-1412, 0407-1413, 0407-1414) Establishment Name Address ID/FEI Business Operations GE Healthcare Ireland Limited 988006565 MANUFACTURE(0407-1412, 0407-1413, 0407-1414) , REPACK(0407-1412, 0407-1413, 0407-1414) , RELABEL(0407-1412, 0407-1413, 0407-1414) Establishment Name Address ID/FEI Business Operations GE Healthcare Lindesnes 518890970 API MANUFACTURE(0407-1412, 0407-1413, 0407-1414) Establishment Name Address ID/FEI Business Operations GE Healthcare AS 515048908 MANUFACTURE(0407-1412, 0407-1413, 0407-1414) , REPACK(0407-1412, 0407-1413, 0407-1414) , RELABEL(0407-1412, 0407-1413, 0407-1414)
Trademark Results [OMNIPAQUE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 OMNIPAQUE 73340246 1207823 Live/Registered |
Sterling Drug Inc. 1981-12-07 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.