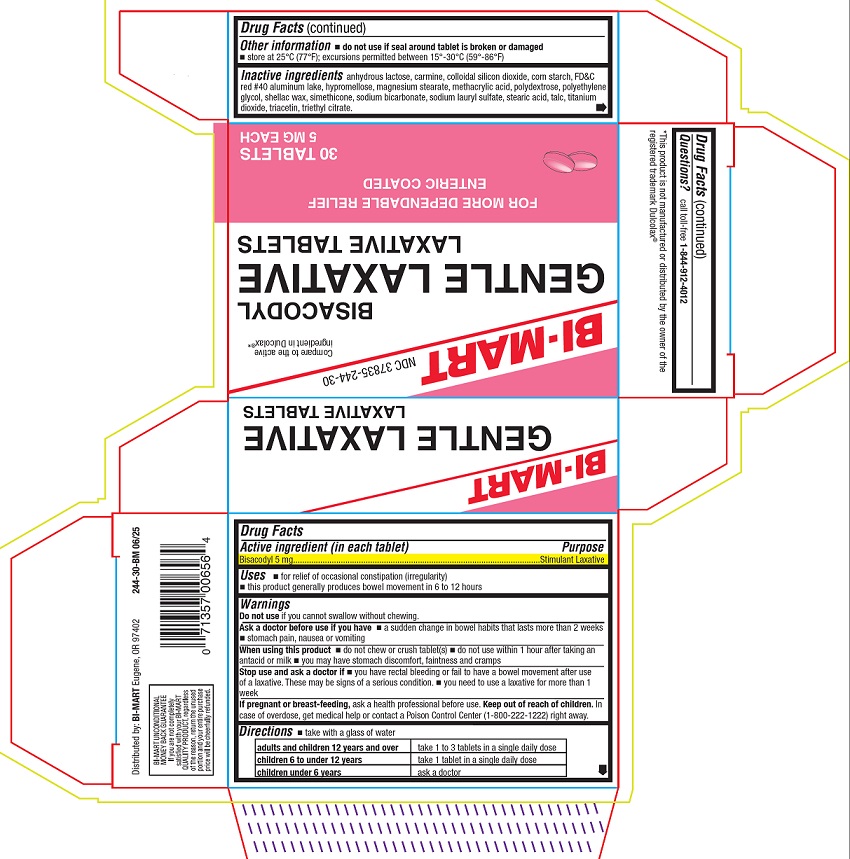
GENTLE LAXATIVE- bisacodyl tablet, delayed release
Gentle Laxative by
Drug Labeling and Warnings
Gentle Laxative by is a Otc medication manufactured, distributed, or labeled by Bi-Mart, LNK International, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
- a sudden change in bowel habits that lasts more than 2 weeks
- stomach pain, nausea or vomiting
When using this product
- do not chew or crush tablet(s)
- do not use within 1 hour after taking an antacid or milk
- you may have stomach discomfort, faintness, and cramps
- Directions
- Other information
-
Inactive ingredients
anhydrous lactose, carmine, colloidal silicon dioxide, corn starch, FD&C red #40 aluminum lake, hypromellose, magnesium stearate, methacrylic acid, polydextrose, polyethylene glycol, shellac wax, simethicone, sodium bicarbonate, sodium lauryl sulfate, stearic acid, talc, titanium dioxide, triacetin, triethyl citrate.
- Questions?
- Principal display panel
-
INGREDIENTS AND APPEARANCE
GENTLE LAXATIVE
bisacodyl tablet, delayed releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 37835-244 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BISACODYL (UNII: 10X0709Y6I) (DEACETYLBISACODYL - UNII:R09078E41Y) BISACODYL 5 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STARCH, CORN (UNII: O8232NY3SJ) FD&C RED NO. 40 ALUMINUM LAKE (UNII: 6T47AS764T) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) MAGNESIUM STEARATE (UNII: 70097M6I30) METHACRYLIC ACID (UNII: 1CS02G8656) POLYDEXTROSE (UNII: VH2XOU12IE) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) SHELLAC (UNII: 46N107B71O) DIMETHICONE (UNII: 92RU3N3Y1O) SODIUM BICARBONATE (UNII: 8MDF5V39QO) SODIUM LAURYL SULFATE (UNII: 368GB5141J) STEARIC ACID (UNII: 4ELV7Z65AP) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) Product Characteristics Color pink Score no score Shape ROUND Size 8mm Flavor Imprint Code B Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 37835-244-30 2 in 1 CARTON 08/01/2025 1 15 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 08/01/2025 Labeler - Bi-Mart (027630078) Registrant - Bi-Mart (027630078) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 117025878 manufacture(37835-244)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.