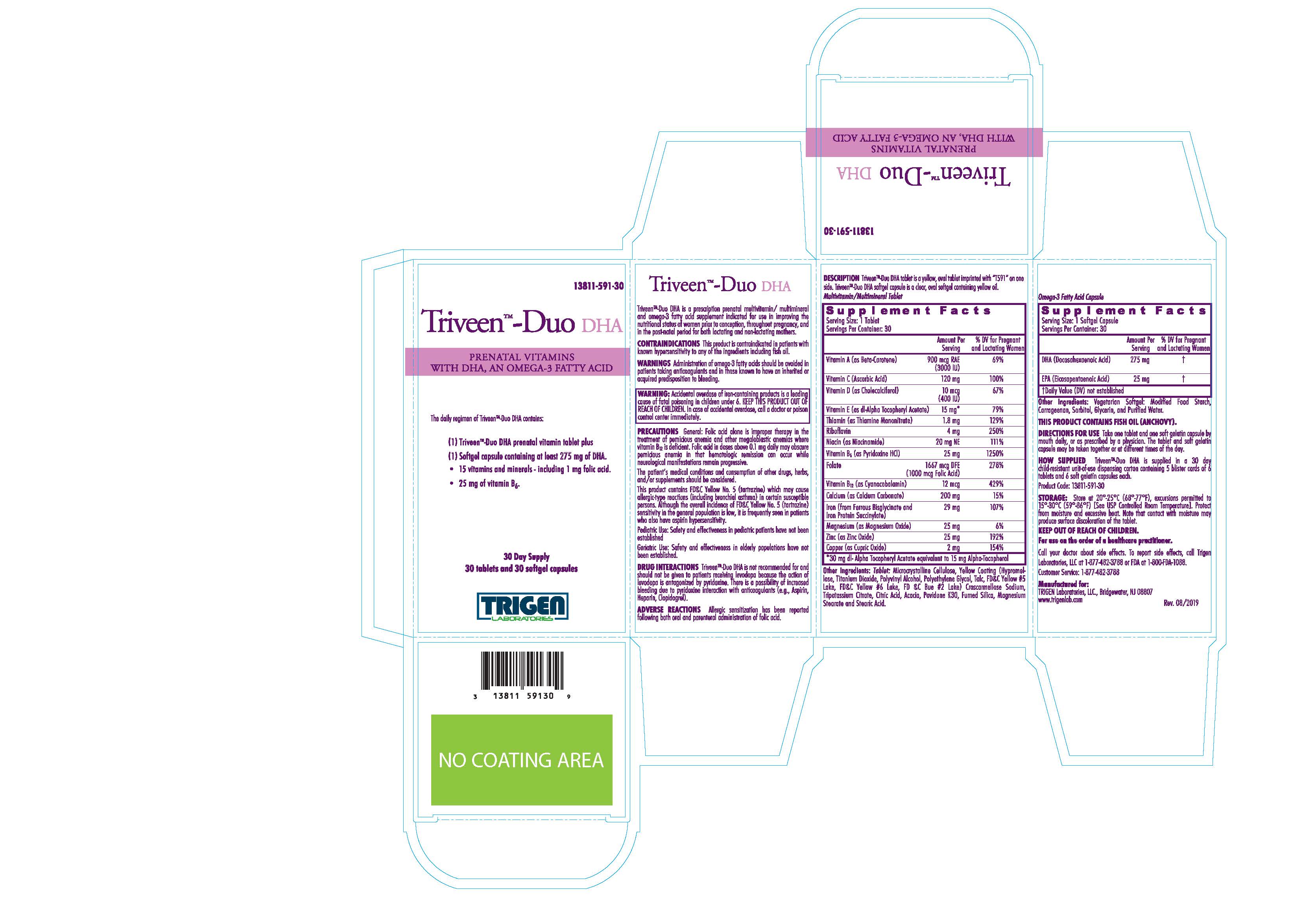
TRIVEEN-DUO DHA- .beta.-carotene, ascorbic acid, cholecalciferol, .alpha.-tocopheryol acetate-dl, thiamine mononitrate, riboflavin, niacinamide, pyridoxine hydrochloride, folic acid, cyanocobalamin, calcium carbonate, ferrous bisglycinate, iron protein succinylate, magnesium oxide, zinc oxide, cupric oxide, and doconexent kit
Triveen-Duo DHA by
Drug Labeling and Warnings
Triveen-Duo DHA by is a Other medication manufactured, distributed, or labeled by Trigen Laboratories, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
SUPPLEMENT FACTS
Multivitamin/Multimineral Tablet
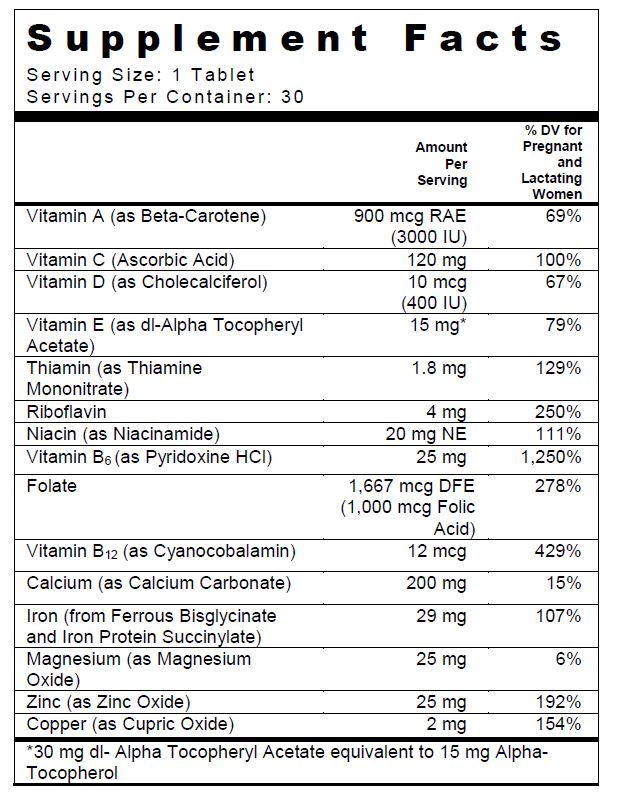
Other Ingredients: Tablet: Microcrystalline Cellulose, Yellow Coating (Hypromellose, Titanium Dioxide, Polyvinyl Alcohol, Polyethylene Glycol,Talc, FD&C Yellow #5 Lake, FD&C Yellow #6 Lake, FD&C Blue #2 Lake) Croscarmellose Sodium, Tripotassium Citrate, Citric Acid, Acacia, Povidone K30, Fumed Silica, Magnesium Stearate and Stearic Acid.
Omega-3 Fatty Acid Capsule
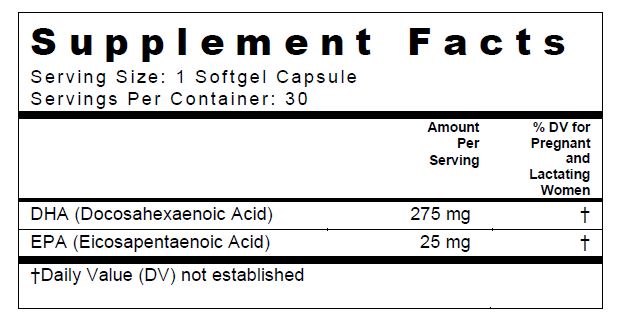
Other Ingredients: Vegetarian Softgel: Modified Food Starch, Carrageenan, Sorbitol, Glycerin, and Purified Water.
THIS PRODUCT CONTAINS FISH OIL (ANCHOVY).
Triveen™-Duo DHA is a prescription prenatal multivitamin/ multimineral and omega-3 fatty acid supplement indicated for use in improving the nutritional status of women prior to conception, throughout pregnancy, and in the post-natal period for both lactating and non-lactating mothers. - CONTRAINDICATIONS
-
WARNINGS
Administration of omega-3 fatty acids should be avoided in patients taking anticoagulants and in those known to have an inherited or acquired predisposition to bleeding.
WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. Keep this product out of reach of children. In case of accidental overdose, call a doctor or poison control center immediately. -
PRECAUTIONS
General: Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B12 is deficient. Folic acid in doses above 0.1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations remain progressive.
The patient’s medical conditions and consumption of other drugs, herbs, and/or supplements should be considered.
This product contains FD&C Yellow No. 5 (tartrazine) which may cause allergic-type reactions (including bronchial asthma) in certain susceptible persons. Although the overall incidence of FD&C Yellow No. 5 (tartrazine) sensitivity in the general population is low, it is frequently seen in patients who also have aspirin hypersensitivity.
Pediatric Use: Safety and effectiveness in pediatric patients have not been established
Geriatric Use: Safety and effectiveness in elderly populations have not been established. - DRUG INTERACTIONS
- ADVERSE REACTIONS
- DESCRIPTION
- DIRECTIONS FOR USE
- HOW SUPPLIED
- STORAGE
-
HEALTH CLAIM
KEEP OUT OF REACH OF CHILDREN.
For use on the order of a healthcare practitioner.
Call your doctor about side effects. To report side effects, call Trigen Laboratories, LLC at 1-877-482-3788 or FDA at 1-800-FDA-1088.
Customer Service: 1-877-482-3788 Rev. 08/2019
Manufactured for:
Trigen Laboratories, LLC
Bridgewater, NJ 08807
www.trigenlab.com - PRINCIPAL DISPLAY PANEL - Kit Carton
-
INGREDIENTS AND APPEARANCE
TRIVEEN-DUO DHA
.beta.-carotene, ascorbic acid, cholecalciferol, .alpha.-tocopheryol acetate-dl, thiamine mononitrate, riboflavin, niacinamide, pyridoxine hydrochloride, folic acid, cyanocobalamin, calcium carbonate, ferrous bisglycinate, iron protein succinylate, magnesium oxide, zinc oxide, cupric oxide, and doconexent kitProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:13811-591 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:13811-591-30 5 in 1 CARTON 1 1 in 1 BLISTER PACK Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BLISTER PACK 6 Part 2 1 BLISTER PACK 6 Part 1 of 2 TRIVEEN-DUO DHA
.beta.-carotene, ascorbic acid, cholecalciferol, .alpha.-tocopheryol acetate-dl, thiamine mononitrate, riboflavin, niacinamide, pyridoxine hydrochloride, folic acid, cyanocobalamin, calcium carbonate, ferrous bisglycinate, iron protein succinylate, magnesium oxide, zinc oxide, cupric oxide, and doconexent tablet, film coatedProduct Information Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength .BETA.-CAROTENE (UNII: 01YAE03M7J) (.BETA.-CAROTENE - UNII:01YAE03M7J) .BETA.-CAROTENE 3000 [iU] ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 120 mg CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 400 [iU] .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) (.ALPHA.-TOCOPHEROL, DL- - UNII:7QWA1RIO01) .ALPHA.-TOCOPHEROL, DL- 15 mg THIAMINE MONONITRATE (UNII: 8K0I04919X) (Thiamine ION - UNII:4ABT0J945J) THIAMINE 1.8 mg RIBOFLAVIN (UNII: TLM2976OFR) (RIBOFLAVIN - UNII:TLM2976OFR) RIBOFLAVIN 4 mg NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 20 mg PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE HYDROCHLORIDE 25 mg FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 1 mg CYANOCOBALAMIN (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) CYANOCOBALAMIN 12 ug CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CARBONATE 200 mg FERROUS BISGLYCINATE (UNII: SFW1D987QV) (FERROUS CATION - UNII:GW89581OWR) FERROUS CATION 25 mg IRON PROTEIN SUCCINYLATE (UNII: VBJ9L90P4L) (IRON PROTEIN SUCCINYLATE - UNII:VBJ9L90P4L) IRON PROTEIN SUCCINYLATE 4 mg MAGNESIUM OXIDE (UNII: 3A3U0GI71G) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM OXIDE 25 mg ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 25 mg CUPRIC OXIDE (UNII: V1XJQ704R4) (CUPRIC CATION - UNII:8CBV67279L) CUPRIC CATION 2 mg Inactive Ingredients Ingredient Name Strength CASTOR OIL (UNII: D5340Y2I9G) POTASSIUM CITRATE ANHYDROUS (UNII: 86R1NVR0HW) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYPROMELLOSES (UNII: 3NXW29V3WO) ACACIA (UNII: 5C5403N26O) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POVIDONE K30 (UNII: U725QWY32X) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) STEARIC ACID (UNII: 4ELV7Z65AP) POLYSORBATE 80 (UNII: 6OZP39ZG8H) ETHYLCELLULOSES (UNII: 7Z8S9VYZ4B) TALC (UNII: 7SEV7J4R1U) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 5 in 1 CARTON 1 6 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date DIETARY SUPPLEMENT 11/01/2010 Part 2 of 2 TRIVEEN-DUO DHA
.beta.-carotene, ascorbic acid, cholecalciferol, .alpha.-tocopheryol acetate-dl, thiamine mononitrate, riboflavin, niacinamide, pyridoxine hydrochloride, folic acid, cyanocobalamin, calcium carbonate, ferrous bisglycinate, iron protein succinylate, magnesium oxide, zinc oxide, cupric oxide, and doconexent capsuleProduct Information Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOCONEXENT (UNII: ZAD9OKH9JC) (DOCONEXENT - UNII:ZAD9OKH9JC) DOCONEXENT 275 mg Inactive Ingredients Ingredient Name Strength MODIFIED CORN STARCH (1-OCTENYL SUCCINIC ANHYDRIDE) (UNII: 461P5CJN6T) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) CARRAGEENAN (UNII: 5C69YCD2YJ) SORBITOL (UNII: 506T60A25R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 5 in 1 CARTON 1 6 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date DIETARY SUPPLEMENT 11/01/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date DIETARY SUPPLEMENT 11/01/2010 Labeler - Trigen Laboratories, LLC (830479668)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.