JASCAYD- nerandomilast tablet, film coated
JASCAYD by
Drug Labeling and Warnings
JASCAYD by is a Prescription medication manufactured, distributed, or labeled by Boehringer Ingelheim Pharmaceuticals, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use JASCAYD safely and effectively. See full prescribing information for JASCAYD.
JASCAYD® (nerandomilast tablets), for oral use
Initial U.S. Approval: 2025RECENT MAJOR CHANGES
Indications and Usage, Progressive Pulmonary Fibrosis (1.2) 12/2025 INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
- Recommended Dosage: 18 mg orally twice daily approximately 12 hours apart with or without food. (2.1)
- Reduce JASCAYD to 9 mg twice daily for patients who are unable to tolerate 18 mg twice daily, except in patients taking concomitant pirfenidone. (2.1, 7.1)
- Swallow tablets whole or dispersed in water. (2.1)
- See full prescribing information for dosage modification for concomitant use with CYP3A inhibitors and administration instructions for patients who have difficulty swallowing tablets. (2.2, 2.3)
DOSAGE FORMS AND STRENGTHS
Tablets: 9 mg and 18 mg (3)
CONTRAINDICATIONS
None. (4)
ADVERSE REACTIONS
Most common adverse reactions (≥5%) are diarrhea, COVID-19, upper respiratory tract infection, depression, weight decreased, decreased appetite, nausea, fatigue, headache, vomiting, back pain, and dizziness. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Boehringer Ingelheim Pharmaceuticals, Inc. at (800) 542-6257 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 12/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Idiopathic Pulmonary Fibrosis
1.2 Progressive Pulmonary Fibrosis
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Dosage Modification of JASCAYD for Concomitant Use With CYP3A Inhibitors
2.3 Administration Instructions for Patients Who Have Difficulty Swallowing Tablets
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Effects of Other Drugs on JASCAYD
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Idiopathic Pulmonary Fibrosis
14.2 Progressive Pulmonary Fibrosis
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The recommended dosage of JASCAYD is 18 mg twice daily, administered orally (swallow tablets whole or dispersed in water) approximately 12 hours apart, with or without food.
- Reduce JASCAYD to 9 mg twice daily for patients who are unable to tolerate 18 mg twice daily, except in patients who concomitantly use JASCAYD with pirfenidone.
Recommended Dosage for Concomitant Use with Pirfenidone
Recommended dosage of JASCAYD is 18 mg twice daily when used concomitantly with pirfenidone. Do not reduce dosage to 9 mg twice daily [see Drug Interactions (7.1)].
Administration Instructions
Swallow JASCAYD tablets whole or dispersed in water [see Dosage and Administration (2.3) and Clinical Pharmacology (12.3)].
Missed Dose(s)
If a dose of JASCAYD is missed, advise the patient to take the next dose at the next scheduled time. Advise the patient to not make up for a missed dose.
Maximum Recommended Dosage
The maximum recommended dosage of JASCAYD is 18 mg twice daily.
2.2 Dosage Modification of JASCAYD for Concomitant Use With CYP3A Inhibitors
Strong CYP3A Inhibitors
Reduce JASCAYD dosage to 9 mg twice daily when used concomitantly with strong CYP3A inhibitors [see Drug Interactions (7.1)].
Moderate and Weak CYP3A Inhibitors
No dosage modification is recommended for JASCAYD when used concomitantly with moderate or weak CYP3A inhibitors.
2.3 Administration Instructions for Patients Who Have Difficulty Swallowing Tablets
Disperse JASCAYD tablet in water and administer as follows:
- Place approximately 100 mL (3 to 4 ounces) of non-carbonated, room temperature water in a glass. Do not use any other liquids.
- Place a JASCAYD tablet in the water, without crushing, and stir regularly for approximately 15 to 20 minutes until the tablet is dispersed into very small pieces (the tablet will not completely dissolve).
- Drink the dispersion within 2 hours of mixing.
- If the dispersion is not drunk immediately, stir again before drinking.
- Rinse the glass with approximately 100 mL (3 to 4 ounces) of water and drink to ensure the full dose is administered.
-
3 DOSAGE FORMS AND STRENGTHS
Tablets:
- 9 mg, light yellow, oval, biconvex, film-coated tablets debossed with the Boehringer Ingelheim company symbol on one side and "F9" on the other side.
- 18 mg, light red, oval, biconvex, film-coated tablets debossed with the Boehringer Ingelheim company symbol on one side and "F18" on the other side.
- 4 CONTRAINDICATIONS
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in clinical trials of another drug and may not reflect the rates observed in practice.
Adverse Reactions for Idiopathic Pulmonary Fibrosis
The safety of JASCAYD was based on a randomized, placebo-controlled, double-blind trial (FIBRONEER-IPF), which included 1,177 adult patients with IPF who were randomized in a 1:1:1 ratio to receive JASCAYD 9 mg twice daily, JASCAYD 18 mg twice daily, or matching placebo. Patients received JASCAYD or placebo with or without background antifibrotic treatment (nintedanib or pirfenidone) for at least 52 weeks [see Clinical Studies (14.1)]. The median duration of exposure was 14 months in each treatment arm.
Discontinuation due to adverse reactions occurred more frequently in patients treated with JASCAYD (with or without background antifibrotic treatment) 18 mg (15%) and 9 mg (12%) compared to placebo (11%). The most frequent adverse reaction leading to discontinuation of JASCAYD 18 mg and 9 mg was diarrhea (6% and 2%, respectively).
Table 1 lists the most common adverse reactions from the studied population with an incidence of greater than or equal to 5% in JASCAYD-treated patients and more common than the placebo group.
Table 1 Adverse Reactions with JASCAYD with Incidence of ≥5% and More Common than Placebo in Patients1 with IPF (FIBRONEER-IPF Trial) JASCAYD
18 mg BID
n=392JASCAYD
9 mg BID
n=392Placebo
n=3931Studied population including patients who received JASCAYD with or without background antifibrotic treatment (nintedanib or pirfenidone)
2Includes depression, depressed mood, depression rating scale score increased, suicidal ideation, adjustment disorder with depressed mood, depressive symptom
BID: twice daily; COVID-19: infection with SARS-CoV-2 virusDiarrhea 42% 31% 17% COVID-19 13% 16% 12% Upper respiratory tract infection 13% 11% 10% Depression2 12% 11% 10% Weight decreased 11% 10% 8% Decreased appetite 9% 9% 5% Nausea 8% 9% 7% Fatigue 7% 8% 6% Headache 7% 6% 5% Vomiting 6% 5% 5% Back pain 6% 5% 4% Dizziness 5% 6% 5% Specific Adverse Reactions of JASCAYD for IPF with or without Concomitant Use of Nintedanib or Pirfenidone
Diarrhea
Diarrhea was more common in patients using JASCAYD with concomitant nintedanib. In patients taking nintedanib, diarrhea occurred in 62%, 50%, and 28% of patients treated with JASCAYD 18 mg twice daily, JASCAYD 9 mg twice daily, and placebo, respectively. In patients using concomitant pirfenidone, diarrhea occurred in 24% and 8% of patients treated with JASCAYD 18 mg twice daily and placebo, respectively. In patients without concomitant antifibrotic treatment, diarrhea occurred in 26%, 17%, and 8% of patients using JASCAYD 18 mg twice daily, JASCAYD 9 mg twice daily, and placebo, respectively.
Diarrhea was the most common adverse reaction associated with treatment discontinuation, and most common with JASCAYD used concomitantly with nintedanib: discontinuation occurred in 13%, 2%, and 1% of patients treated with JASCAYD 18 mg twice daily, JASCAYD 9 mg twice daily and placebo, respectively. No treatment discontinuations due to diarrhea occurred in patients treated with background pirfenidone and JASCAYD 18 mg twice daily or background pirfenidone with placebo. Diarrhea leading to treatment discontinuation occurred in 1% of patients treated with JASCAYD 18 mg twice daily and in no patients treated with JASCAYD 9 mg or placebo without concomitant antifibrotic treatment.
In most patients treated with JASCAYD, diarrhea was of mild to moderate intensity and generally occurred within the first 3 months of treatment.
Weight Decrease
Weight decrease was most common in patients who received JASCAYD concomitantly with nintedanib in the studied population: in patients taking nintedanib, weight decrease occurred in 16%, 14%, and 12% of patients treated with JASCAYD 18 mg twice daily, JASCAYD 9 mg twice daily, and placebo, respectively. In patients using concomitant pirfenidone, weight decrease occurred in 6% and 5% of patients treated with JASCAYD 18 mg twice daily and placebo, respectively. In patients without background antifibrotic therapy, weight decrease occurred in 8%, 2%, and 6% of patients treated with JASCAYD 18 mg twice daily, JASCAYD 9 mg twice daily, and placebo, respectively.
Decreased Appetite
In patients taking nintedanib, decreased appetite occurred in 7%, 10%, and 4% in JASCAYD 18 mg twice daily, JASCAYD 9 mg twice daily, and placebo, respectively. In patients using concomitant pirfenidone, decreased appetite occurred in 13% and 10% of patients treated with JASCAYD 18 mg twice daily and placebo, respectively. In patients without concomitant antifibrotic treatment, decreased appetite occurred in 9%, 6%, and 0% of patients treated with JASCAYD 18 mg twice daily, JASCAYD 9 mg twice daily, and placebo, respectively.
Less Common Adverse Reactions in IPF
Less common adverse reactions in the IPF population following administration of JASCAYD included asthenia (5% JASCAYD 18 mg twice daily, 4% JASCAYD 9 mg twice daily, and 2% placebo), amylase increased (1% JASCAYD 18 mg twice daily, 1% JASCAYD 9 mg twice daily, and 0% placebo), and vasculitis (1% JASCAYD 18 mg twice daily, 1% JASCAYD 9 mg twice daily, and 0% placebo).
Adverse Reactions for Progressive Pulmonary Fibrosis
The safety of JASCAYD was based on a randomized, placebo-controlled, double-blind trial (FIBRONEER-ILD), in which 1,178 adult patients with PPF were randomized in a 1:1:1 ratio to receive JASCAYD 9 mg twice daily, JASCAYD 18 mg twice daily, or matching placebo. Patients received JASCAYD or placebo with or without background nintedanib treatment for at least 52 weeks [see Clinical Studies (14.2)]. The median duration of exposure was 15 months in each treatment arm.
The most common adverse reactions in patients with PPF treated with JASCAYD were generally consistent with those observed in patients with IPF.
Specific Adverse Reactions of JASCAYD for PPF with or without Concomitant Use of Nintedanib
Diarrhea
Diarrhea was more common in patients using JASCAYD with concomitant nintedanib. In patients taking nintedanib, diarrhea occurred in 49%, 50%, and 37% of patients treated with JASCAYD 18 mg twice daily, JASCAYD 9 mg twice daily, and placebo, respectively. In patients without concomitant use of nintedanib, diarrhea occurred in 27%, 16%, and 16% of patients using JASCAYD 18 mg twice daily, JASCAYD 9 mg twice daily, and placebo, respectively.
Diarrhea was the most common adverse reaction associated with treatment discontinuation, and most common with JASCAYD used concomitantly with nintedanib: discontinuation occurred in 4%, 3%, and 1% in patients treated with JASCAYD 18 mg twice daily, JASCAYD 9 mg twice daily, and placebo, respectively. Diarrhea leading to treatment discontinuation occurred in 1% of patients treated with JASCAYD 18 mg twice daily and in no patients treated with JASCAYD 9 mg or placebo without concomitant use of nintedanib.
In most patients treated with JASCAYD, diarrhea was of mild to moderate intensity and generally occurred within the first 3 months of treatment.
Weight Decrease
Weight decrease was more common in patients who received JASCAYD concomitantly with nintedanib, which occurred in 12%, 10%, and 9% of patients treated with JASCAYD 18 mg twice daily, JASCAYD 9 mg twice daily, and placebo, respectively. In patients without concomitant use of nintedanib, weight decrease occurred in 13%, 8%, and 5% of patients treated with JASCAYD 18 mg twice daily, JASCAYD 9 mg twice daily, and placebo, respectively.
Malignancies
Among patients with PPF, neoplasms (benign, malignant, or unspecified) were reported in more patients with PPF treated with JASCAYD than placebo over the entire study duration of FIBRONEER-ILD (5% JASCAYD 18 mg, 5% JASCAYD 9 mg, and 3% placebo). Malignancies such as non-melanoma skin cancers and small cell lung cancer were observed in patients who received JASCAYD (basal cell carcinoma: JASCAYD 18 mg n=2 [1%] vs. JASCAYD 9 mg n=3 [1%] vs. placebo n=1 [0.3%]; squamous cell carcinoma of skin: JASCAYD 18 mg n=4 [1%] vs. JASCAYD 9 mg n=2 [0.5%] vs. placebo n=0; and small cell lung cancer: JASCAYD 18 mg n=4 [1%] vs. JASCAYD 9 mg and placebo n=0).
Less Common Adverse Reaction(s) in PPF
Less common adverse reactions in the PPF population following administration of JASCAYD include atrial fibrillation (3% JASCAYD 18 mg twice daily, 2% JASCAYD 9 mg twice daily, and <1% placebo).
-
7 DRUG INTERACTIONS
7.1 Effects of Other Drugs on JASCAYD
Strong CYP3A Inhibitors
Reduce the dosage of JASCAYD to 9 mg twice daily when used concomitantly with strong CYP3A inhibitors [see Dosage and Administration (2.2)].
Nerandomilast is a CYP3A substrate. Concomitant use of JASCAYD with a strong CYP3A inhibitor increases exposure of nerandomilast, which may increase the risk of JASCAYD adverse reactions [see Clinical Pharmacology (12.3)].
Moderate or Strong CYP3A Inducers
Avoid use of JASCAYD with strong or moderate CYP3A inducers.
Nerandomilast is a CYP3A substrate. Concomitant use of JASCAYD with moderate or strong CYP3A inducers is expected to decrease exposure of nerandomilast, which may decrease the efficacy of JASCAYD [see Clinical Pharmacology (12.3)].
Pirfenidone
Recommended dosage of JASCAYD is 18 mg twice daily when used concomitantly with pirfenidone. Do not reduce the dosage to 9 mg twice daily [see Dosage and Administration (2.1)].
Concomitant use of JASCAYD with pirfenidone decreases exposure of nerandomilast [see Clinical Pharmacology (12.3)]. When JASCAYD was used concomitantly with pirfenidone in patients with IPF in FIBRONEER-IPF, efficacy was not observed with the JASCAYD 9 mg twice daily dosage [see Clinical Studies (14.1)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on JASCAYD use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage or other adverse maternal or fetal outcomes. There are maternal and fetal risks associated with untreated idiopathic pulmonary fibrosis (IPF) and progressive pulmonary fibrosis (PPF) during pregnancy (Clinical Considerations).
Based on findings from animal reproduction studies, JASCAYD may increase the risk for fetal loss. In an embryo-fetal development study in rats, oral administration of nerandomilast to pregnant rats during organogenesis at an exposure approximately 5 times the maximum recommended human dose (MRHD) of 36 mg/day resulted in an increase in embryo-fetal losses (see Data). Advise pregnant women and females of reproductive potential of the potential risk of fetal loss.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects is 2% to 4% and miscarriage in clinically recognized pregnancies is 15% to 20%.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk
Untreated IPF or PPF can lead to respiratory failure and mortality in the mother and intrauterine growth restriction, preterm birth, fetal hypoxia, and neonatal death.
Animal Data
In an embryo-fetal development study in pregnant rats dosed by the oral route during the period of organogenesis from gestation days 6 to 17, nerandomilast caused an increase in embryo-fetal losses (pre- and post-implantation loss and decreased mean number of live fetuses) at an exposure that was approximately 5 times the MRHD (on an AUC basis with a maternal oral dose of 6 mg/kg/day). Maternal toxicity, as evidenced by decreased body weight gains and adverse clinical signs, was observed at exposures approximately 7 times the MRHD (on an AUC basis with a maternal oral dose of 9 mg/kg/day). No fetal or maternal toxicities were observed at exposures up to 3 and 5 times the MRHD (on an AUC basis with maternal oral doses of 3 mg/kg/day and 6 mg/kg/day), respectively.
In an embryo-fetal development study in pregnant rabbits dosed by the oral route during the period of organogenesis from gestation days 7 to 19, no effects on maternal or fetal development were observed at an exposure that was approximately 4 times the MRHD (on an AUC basis with a maternal oral dose of 15 mg/kg/day).
In a prenatal and postnatal development study in pregnant rats dosed by the oral route during the periods of gestation and lactation from gestation day 6 to lactation day 20, nerandomilast had no effects on delivery or the growth and development of offspring at an exposure that was approximately 2 times the MRHD (on an AUC basis with a maternal oral dose of 3 mg/kg/day).
8.2 Lactation
Risk Summary
There are no data on the presence of nerandomilast or its metabolite in human milk, the effects on the breastfed infant, or the effects on milk production. Nerandomilast is present in animal milk. When a drug is present in animal milk, it is likely that the drug will be present in human milk (see Data).
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for JASCAYD and any potential adverse effects on the breastfed infant from JASCAYD or from the underlying maternal condition.
In a prenatal and postnatal development study in pregnant rats dosed by the oral route during the periods of gestation and lactation from gestation day 6 to lactation day 20, nerandomilast was present in the plasma of rat pups during the lactation period. In a single dose milk secretion study in lactating rats dosed with radiolabeled nerandomilast by the oral route, similar concentrations of total radioactivity were observed in the milk and plasma of lactating females, with the maximum radioactive concentration observed at 1 hour post dose that was significantly reduced by 24 hours post dose. The concentration of total radioactivity in animal milk does not necessarily predict the concentration of drug in human milk.
8.4 Pediatric Use
The safety and effectiveness of JASCAYD for the treatment of idiopathic pulmonary fibrosis or progressive pulmonary fibrosis have not been established in pediatric patients.
8.5 Geriatric Use
There were 930 patients 65 years of age and older in the FIBRONEER-IPF trial [see Clinical Studies (14.1)]. Of the total number of JASCAYD-treated patients with idiopathic pulmonary fibrosis in this trial, 623 (79%) were 65 years of age and older, while 251 (32%) were 75 years of age and older.
There were 733 patients 65 years of age and older in the FIBRONEER-ILD trial [see Clinical Studies (14.2)]. Of the total number of JASCAYD-treated patients with progressive pulmonary fibrosis in this trial, 490 (63%) were 65 years of age and older, while 150 (19%) were 75 years of age and older.
No overall differences in safety or effectiveness of JASCAYD have been observed between patients 65 years of age and older and younger adult patients.
8.6 Renal Impairment
JASCAYD has not been investigated in patients with end stage renal disease (eGFR <15 mL/min/1.73 m2). Use of JASCAYD is not recommended in patients with end stage renal disease (eGFR <15 mL/min/1.73 m2).
The recommended dosage in patients with mild (eGFR ≥60 to <90 mL/min/1.73 m2 according to CKD-EPI), moderate (eGFR ≥30 to <60 mL/min/1.73 m2), or severe renal impairment (eGFR ≥15 to <30 mL/min/1.73 m2) is the same as that in patients with normal renal function [see Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
JASCAYD has not been investigated in patients with severe (Child-Pugh Class C) hepatic impairment. Use of JASCAYD is not recommended in patients with severe (Child-Pugh Class C) hepatic impairment.
The recommended dosage in patients with mild (Child-Pugh Class A) or moderate (Child-Pugh Class B) hepatic impairment is the same as that in patients with normal hepatic function [see Clinical Pharmacology (12.3)].
- 10 OVERDOSAGE
-
11 DESCRIPTION
JASCAYD (nerandomilast tablets), for oral administration, contain nerandomilast, a phosphodiesterase 4 (PDE4) inhibitor. The chemical name of nerandomilast is [1-[[(5R)-2-[4-(5-chloropyrimidin-2-yl)-1-piperidyl]-5-oxo-6,7-dihydrothieno[3,2-d]pyrimidin-4-yl]amino]cyclobutyl]methanol. Nerandomilast has one chiral center and is the R-stereoisomer. Its structural formula is:
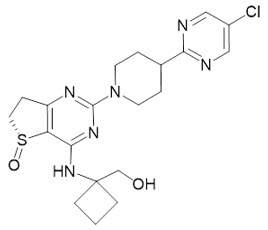
Nerandomilast is a white to off-white to light yellow powder with an empirical formula of C20H25ClN6O2S and a molecular weight of 449 g/mol. At or below pH 3, nerandomilast is very slightly to slightly soluble in water, and above pH 3 it is practically insoluble.
Each film-coated tablet of JASCAYD contains 9 mg or 18 mg of nerandomilast and the following inactive ingredients: croscarmellose sodium, ferric oxide red (18 mg tablets only), ferric oxide yellow (9 mg tablets only), ferrosoferric oxide (18 mg tablets only), hydroxypropyl cellulose, hypromellose, lactose monohydrate, magnesium stearate, mannitol, microcrystalline cellulose, polyethylene glycol, and talc. Each JASCAYD tablet contains less than 5 mg of sodium.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Nerandomilast is an inhibitor of phosphodiesterase 4 (PDE4) with at least nine-fold preferential inhibition of the PDE4B isoenzyme over PDE4A, PDE4C, and PDE4D based on in vitro data. PDE4 hydrolyzes and inactivates cyclic adenosine monophosphate (cAMP). Nerandomilast exerts both antifibrotic and immunomodulatory effects as PDE4B inhibition elevates intracellular cAMP levels and reduces the expression of profibrotic growth factors and inflammatory cytokines, which are overexpressed in IPF and PPF.
12.2 Pharmacodynamics
Exposure-Response
Increases in nerandomilast steady state trough concentrations were associated with better efficacy, as indicated by a smaller reduction in forced vital capacity (FVC) from baseline over 52 weeks.
Cardiac Electrophysiology
At 2.1 times the maximal concentration provided by the maximum recommended dose of nerandomilast, clinically significant QTc interval prolongation was not observed.
12.3 Pharmacokinetics
Nerandomilast exposure increased in a dose proportional manner following administration of single doses of 0.0032 to 2.6 times a single dose of 18 mg and multiple doses of 0.056 to 1 times the maximum recommended dose of 18 mg twice daily. Following administration of multiple doses of 18 mg twice daily, Cmax increases 1.3-fold and AUCtau increases 1.38-fold. Nerandomilast steady state is reached in approximately 4 days.
No clinically relevant differences in nerandomilast pharmacokinetics were observed between healthy subjects, patients with IPF, and patients with PPF.
Absorption
The absolute oral bioavailability of nerandomilast is 73%. Nerandomilast median (min, max) time to maximum plasma concentration (Tmax) is 1 to 1.25 hours (range 0.5 to 4 hours).
Effect of Food
No clinically relevant differences in nerandomilast pharmacokinetics were observed following administration with a high-fat meal (1000 calories, 50% fat).
Distribution
Nerandomilast steady state apparent (oral) central volume of distribution is 93 L (CV 37%). In vitro, nerandomilast plasma protein binding is 77% and is not concentration dependent. Nerandomilast blood-to-plasma ratio is approximately 0.6 to 0.8.
Elimination
The geometric mean (CV%) elimination half-life of nerandomilast is 17 hours (46%) with an apparent (oral) clearance of 15.2 L/h.
Metabolism
Nerandomilast is primarily metabolized by CYP3A and to a lesser extent by multiple UGT enzymes.
After oral administration of nerandomilast, chiral inversion from the pharmacologically active R-enantiomer to the pharmacologically inactive S-enantiomer occurs via metabolism. The S-enantiomer was identified as a minor metabolite of nerandomilast. The R-enantiomer is the predominant circulating enantiomer.
Excretion
After a single oral dose of radiolabeled nerandomilast, approximately 58% of the dose was recovered in feces (14% as unchanged nerandomilast) and 36% of the dose was recovered in urine (13% as unchanged nerandomilast).
Specific Populations
No clinically significant differences in the pharmacokinetics of nerandomilast were observed based on age (range: 18 to 90 years), body weight (range: 31 to 143 kg), sex, race (63% White and 35% Asian), mild (eGFR ≥60 to <90 mL/min/1.73 m2 [calculated according to Chronic Kidney Disease Epidemiology Collaboration]), moderate (eGFR ≥30 to <60 mL/min/1.73 m2), or severe renal impairment (eGFR ≥15 to <30 mL/min/1.73 m2), or mild (Child-Pugh Class A) or moderate (Child-Pugh Class B) hepatic impairment.
Subjects with end stage renal disease or severe hepatic impairment (Child-Pugh Class C) have not been studied [see Use in Specific Populations (8.6, 8.7)].
Drug Interaction Studies
Strong CYP3A Inhibitors: Nerandomilast Cmax increased by 1.3-fold and AUC increased by 2.2-fold following concomitant administration with itraconazole (a strong CYP3A and P-gp inhibitor) 200 mg oral solution once daily for 4 days [see Drug Interactions (7.1)].
Pirfenidone: Nerandomilast trough concentrations at steady state (Ctrough,ss) decreased by approximately 50% following concomitant administration with pirfenidone [see Drug Interactions (7.1)].
Other Drugs: No clinically significant differences in nerandomilast Ctrough,ss were observed when used concomitantly with nintedanib. No clinically significant differences in the pharmacokinetics of the following drugs were observed when used concomitantly with nerandomilast: oral midazolam (CYP3A4 substrate), pirfenidone, and nintedanib.
In Vitro Studies
CYP450 Enzymes: Nerandomilast is a CYP3A4 substrate. Nerandomilast does not inhibit CYP3A4, CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, and CYP2D6.
Transporter Systems: Nerandomilast is a P-glycoprotein (P-gp) substrate, but is not a substrate of BCRP, OATP1B1, OATP1B3, OAT1, OAT3, and OCT2. Nerandomilast is an inhibitor of P-gp, MATE1, MATE2-K, but is not expected to cause clinically significant interactions. Nerandomilast is not an inhibitor of BCRP, OAT1, OAT3, OCT2, OATP1B1, or OATP1B3.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
The carcinogenic potential of nerandomilast was assessed in Tg.rasH2 mice and Wistar Han rats. No evidence of tumorigenicity was observed in male and female Tg.rasH2 mice that received nerandomilast for 26 weeks at oral doses up to 60 and 100 mg/kg/day, respectively. No evidence of tumorigenicity was observed in male or female rats that received nerandomilast for up to 102 weeks at oral doses up to 2 mg/kg/day (approximately 2 times the MRHD on an AUC basis).
Mutagenesis
Nerandomilast was not mutagenic or clastogenic in the following assays: in vitro bacterial reverse mutation (Ames) assay, in vitro chromosomal aberration assay in human peripheral blood lymphocytes, and in vivo rat micronucleus assay.
Impairment of Fertility
Nerandomilast had no effect on fertility in male or female rats at oral doses up to 6 mg/kg/day (approximately 3 or 4 times the MRHD on an AUC basis, respectively). Decreased mating, pregnancy, and fertility indices at the highest tested dose of 9 mg/kg/day (approximately 4 or 9 times the MRHD on an AUC basis) were attributed to excessive general toxicity.
Sexually mature female monkeys administered nerandomilast by the oral route for 39 weeks showed sporadic menstrual cycle prolongation at dose levels of 10 mg/kg/day and 30 mg/kg/day (approximately 3- and 10 times the MRHD on an AUC basis, respectively). Menstrual cycles were not affected in monkeys at oral doses of 3 mg/kg/day (equivalent to the MRHD on an AUC basis).
-
14 CLINICAL STUDIES
14.1 Idiopathic Pulmonary Fibrosis
The efficacy of JASCAYD for idiopathic pulmonary fibrosis (IPF) was evaluated in two randomized, double-blind, placebo-controlled trials (FIBRONEER-IPF [NCT05321069] and Trial 2 [NCT04419506]).
FIBRONEER-IPF enrolled a total of 1,177 adult patients with IPF with or without background antifibrotic treatments (nintedanib or pirfenidone). They were randomized in a 1:1:1 ratio to receive JASCAYD 9 mg twice daily, JASCAYD 18 mg twice daily, or placebo twice daily until the last patient received treatment for 52 weeks (blinded trial duration up to 91 weeks; end of trial duration up to 109 weeks). Randomization was stratified by the presence or absence of background antifibrotic treatments (nintedanib or pirfenidone) at baseline. The FIBRONEER-IPF trial consisted of 83% male and 17% female patients with a mean age of 70 years (age range: 42 to 90 years). The trial population included 68% White, 32% Asian, and <1% Black or African American. For ethnicity, 8% of patients identified as Hispanic or Latino. At baseline, the mean FVC was 78% of predicted normal; 78% of the patients were on stable antifibrotic treatment (nintedanib 46%, pirfenidone 32%) and 22% were not on either treatment (15% treatment naïve, 7% previously discontinued treatment).
Trial 2 was a 12-week trial that enrolled a total of 147 adult patients with IPF with or without background antifibrotic treatments (nintedanib or pirfenidone) and were randomized 2:1 to receive JASCAYD 18 mg twice daily or placebo twice daily for 12 weeks. Randomization was stratified by the presence or absence of background antifibrotic treatments (nintedanib or pirfenidone) at baseline. The trial population in Trial 2 consisted of 77% male, and mean age of 70 years (age range: 40 to 85 years). The trial population included 78% White and 22% Asian. For ethnicity, 9% of patients identified as Hispanic or Latino. At baseline, the mean FVC was 78% of predicted normal; 50% of the patients were on stable antifibrotic treatment (nintedanib 29%, pirfenidone 21%).
In both the FIBRONEER-IPF trial and Trial 2, patients were required to have a diagnosis of IPF based on ATS/ERS/JRS/ALAT criteria. Diagnosis was confirmed by the investigator based on chest high-resolution computed tomography (HRCT) scan and, if available, lung biopsy, and usual interstitial pneumonia (UIP) or probable UIP HRCT pattern consistent with the clinical diagnosis of IPF. Patients were also required to be greater than or equal to 40 years of age with an FVC greater than or equal to 45% of predicted and a carbon monoxide diffusing capacity (DLCO, corrected for hemoglobin) greater than or equal to 25% of predicted. Prior to Visit 1 and during screening, patients had to be on stable treatment with nintedanib or pirfenidone (no dose changes for at least 12 weeks) and planned to stay on this background antifibrotic treatment after randomization. Alternatively, patients were required to be naïve to or have previously discontinued nintedanib or pirfenidone for at least 8 weeks and did not plan to start or re-start background antifibrotic treatment. Patients with active vasculitis, severe depression or suicidal behavior or ideation, or use of immunomodulatory medications (other than prednisone ≤15 mg/day or equivalent) were excluded.
Change from Baseline in Forced Vital Capacity
The primary endpoint in FIBRONEER-IPF trial was the absolute change from baseline in forced vital capacity (FVC) in milliliters (mL) at 52 weeks for JASCAYD compared with placebo.
In the FIBRONEER-IPF trial population, there was less decline in absolute change from baseline in FVC in patients who received JASCAYD compared with patients who received placebo (accounting for mortality), and this reduction in decline was statistically significant. The adjusted mean decline in patients receiving JASCAYD 18 mg or JASCAYD 9 mg was -106 mL and -122 mL, respectively, whereas in the placebo group, an adjusted mean decline of -170 mL was observed. The respective treatment differences compared with the placebo group were 64 mL (95% CI: 25, 102) and 48 mL (95% CI: 10, 86).
In the FIBRONEER-IPF trial, the results of the primary endpoint across subgroups by background antifibrotic treatment (nintedanib, pirfenidone, or none) for JASCAYD 18 mg versus placebo were consistent with the overall population. Efficacy was not observed in patients who received JASCAYD 9 mg twice daily with pirfenidone as background antifibrotic treatment [see Drug Interactions (7.1)].
Figure 1 shows the change in FVC from baseline over time in patients who received JASCAYD 18 mg twice daily compared to placebo in the FIBRONEER-IPF trial.
Display based on descriptive means and standard errors for change from baseline values from patients with results that were available at each week.
bid = twice dailyFigure 1 Change from Baseline in FVC (mL) in Adults with IPF over 52 weeks with JASCAYD 18 mg Compared to Placebo (FIBRONEER-IPF) 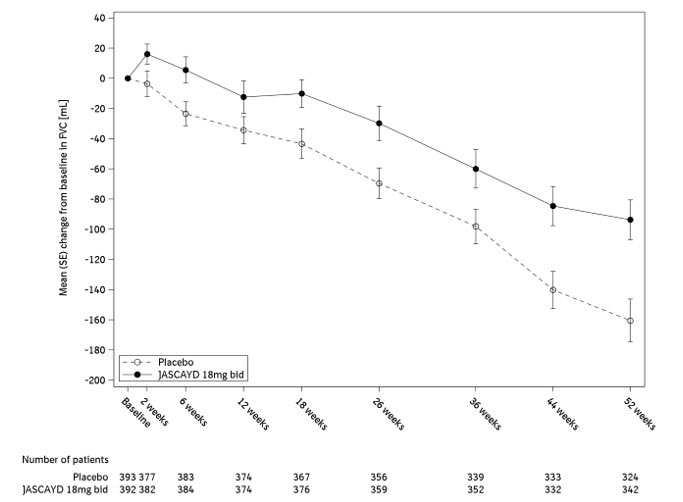
In Trial 2, patients taking JASCAYD 18 mg twice daily compared to placebo, with or without background antifibrotic treatments, had a reduction in FVC decline at Week 12 of 91 mL (95% CI: 44, 138).
Time To First Acute IPF Exacerbation, First Hospitalization for Respiratory Cause, Or Death
The key secondary endpoint in the FIBRONEER-IPF trial was time to first occurrence of any of the components of the composite endpoint over the blinded duration of the trial (up to 91 weeks): acute IPF exacerbation, hospitalization for respiratory cause, or death. Acute IPF exacerbation was defined as acute worsening or development of dyspnea typically less than one month duration, computed tomography with new bilateral ground-glass opacity and/or consolidation superimposed on a background pattern consistent with IPF, and deterioration not fully explained by cardiac failure or fluid overload. Neither acute IPF exacerbations nor respiratory hospitalizations were adjudicated.
Overall, there was no statistically significant treatment difference in hazard ratio (HR) for the JASCAYD 18 mg or 9 mg groups compared to placebo for the key secondary composite endpoint (JASCAYD 18 mg and 9 mg, respectively: HR 1.17 [95% CI: 0.86, 1.59] and HR 1.03 [95% CI: 0.75, 1.41]).
Survival
In the FIBRONEER-IPF trial population, the hazard ratio for all-cause mortality, assessed until the end of trial (up to 109 weeks), did not show a significant treatment difference for JASCAYD 18 mg or 9 mg compared to placebo (HR: 0.66 [95% CI: 0.41, 1.08] and HR 0.95 [95% CI: 0.61, 1.49], respectively).
Figure 2 shows the cumulative incidence of death in adults with IPF in the FIBRONEER-IPF trial for JASCAYD 18 mg and placebo.
bid = twice daily Figure 2 Cumulative Incidence of Death over the Duration of the Trial in Adults with IPF (FIBRONEER-IPF) 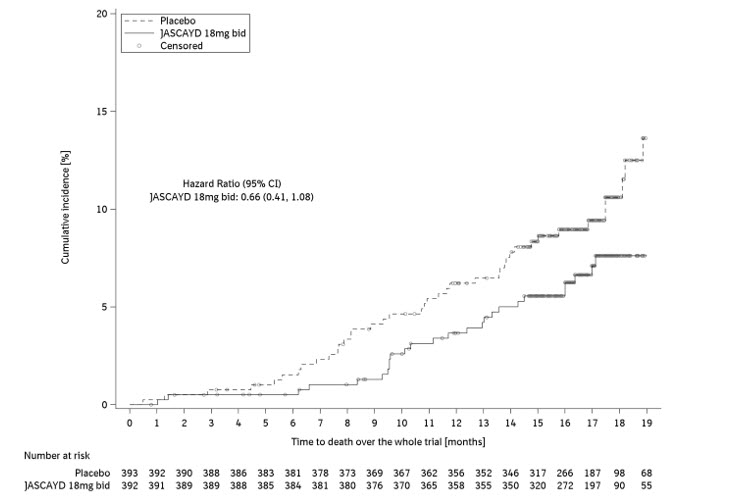
14.2 Progressive Pulmonary Fibrosis
The efficacy of JASCAYD for progressive pulmonary fibrosis (PPF) was evaluated in a randomized, double-blind, placebo-controlled trial (FIBRONEER-ILD [NCT05321082]).
FIBRONEER-ILD enrolled 1,178 adult patients with PPF with or without background treatment with nintedanib. They were randomized in a 1:1:1 ratio to receive JASCAYD 9 mg twice daily, JASCAYD 18 mg twice daily, or placebo twice daily until the last patient received treatment for 52 weeks (blinded trial duration up to 109 weeks; end of trial duration up to 114 weeks). Randomization was stratified by the presence or absence of nintedanib therapy and by high resolution computed tomography (HRCT) pattern (UIP or UIP-like fibrotic pattern vs Other fibrotic patterns) using central review. The FIBRONEER-ILD trial consisted of 56% male and 44% female with a mean age of 66 years (age range: 26 to 88 years). The trial population included 58% White, 38% Asian, 1% Black or African American, 1% American Indian or Alaska Native, and 1% Multiple. For ethnicity, 14% of patients identified as Hispanic or Latino. At baseline, the mean FVC was 70% of predicted normal; 44% of the patients were on stable treatment with nintedanib and 56% of the patients were not treated with nintedanib (44% of patients were treatment naïve and 12% previously discontinued nintedanib treatment). On baseline HRCT, 71% of the patients had UIP or UIP-like fibrotic pattern and 29% of the patients had other fibrotic patterns. The underlying clinical ILD diagnoses were autoimmune ILDs (28%), hypersensitivity pneumonitis (20%), unclassifiable idiopathic interstitial pneumonia (20%), idiopathic nonspecific interstitial pneumonia (19%), and other ILDs (14%).
Patients with PPF were enrolled if they had relevant fibrosis (greater than 10% fibrotic features) on HRCT and presented with clinical signs of progression (defined as FVC decline greater than or equal to 10%, FVC decline greater than or equal to 5% and less than 10% with worsening of respiratory symptoms or imaging, or worsening of respiratory symptoms and worsening imaging all in the 24 months prior to screening). Patients were required to be greater than or equal to 18 years of age and to have an FVC greater than or equal to 45% of predicted and a DLCO (corrected for hemoglobin) greater than or equal to 25% of predicted. Prior to Visit 1 and during screening, patients had to be on stable therapy with nintedanib (no dose changes for at least 12 weeks) and planned to stay on this background PPF therapy after randomization. Alternatively, patients were required to be naïve to or have previously discontinued nintedanib for at least 8 weeks and did not plan to start or re-start background PPF treatment.
Patients were excluded from the trial if they had a pre-bronchodilator FEV1/FVC less than 0.7, acute ILD exacerbation within 3 months prior to the trial, active vasculitis within 8 weeks prior to the trial, a history of suicidal behavior or ideation, or severe depression. Patients were also excluded if they were being treated with the following medications prior to the trial: prednisone >15 mg/day or equivalent within 4 weeks; cyclophosphamide, tocilizumab, mycophenolate, pirfenidone within 8 weeks; rituximab within 6 months.
Change from Baseline in Forced Vital Capacity
The primary endpoint in FIBRONEER-ILD trial was the absolute change from baseline in forced vital capacity (FVC) in mL at 52 weeks for JASCAYD compared with placebo.
In the FIBRONEER-ILD trial population, there was less decline in absolute change from baseline in FVC in patients who received JASCAYD compared with patients who received placebo (accounting for mortality), and this reduction in decline was statistically significant. The adjusted mean decline in patients receiving JASCAYD 18 mg or JASCAYD 9 mg was -86 mL and -69 mL, respectively, whereas in the placebo group, an adjusted mean decline of -152 mL was observed. The respective treatment difference compared with the placebo group was 65 mL (95% CI: 30, 101) and 83 mL (95% CI: 48, 118). FVC results across relevant subgroups were similar. See Figure 3.
These results are from an exploratory analysis. Values shown here are for descriptive purposes. Includes values from patients with results at Week 52, last observed value for patients who had a lung transplant or died before Week 52, and imputed Week 52 values for all other patients with missing data.
bid = twice dailyFigure 3 Absolute Change from Baseline in FVC (mL) at 52 Weeks for JASCAYD 18 mg versus Placebo (FIBRONEER-ILD) 
Figure 4 shows the change in FVC from baseline over time patients receiving JASCAYD 18 mg twice daily compared to placebo in the FIBRONEER-ILD trial.
Display based on descriptive means and standard errors for change from baseline values from patients with results that were available at each week.
bid = twice dailyFigure 4 Change from Baseline in FVC (mL) in Adults with PPF over 52 Weeks with JASCAYD 18 mg Compared to Placebo (FIBRONEER-ILD) 
Time To First Acute ILD Exacerbation, First Hospitalization for Respiratory Cause, Or Death
The key secondary endpoint in the FIBRONEER-ILD trial was the time to the first occurrence of any of the components of the composite endpoint over the blinded duration of the trial (up to 109 weeks): acute ILD exacerbation, hospitalization for respiratory cause, or death. Acute ILD exacerbation was defined as acute worsening or development of dyspnea typically less than 1 month duration, computed tomography with new bilateral ground-glass opacity and/or consolidation superimposed on a background pattern consistent with fibrosing ILD, and deterioration not fully explained by cardiac failure or fluid overload. Neither acute ILD exacerbations nor respiratory hospitalizations were adjudicated.
Overall, there was no statistically significant treatment difference in the hazard ratio (HR) for the JASCAYD 18 mg or 9 mg groups compared to placebo for the key secondary composite endpoint (JASCAYD 18 mg or 9 mg groups, respectively, compared to placebo: HR 0.77 (95% CI: 0.59, 1.01) and HR 0.88 (95% CI: 0.68, 1.14).
See Figure 5 for results of JASCAYD 18 mg versus placebo for the key secondary endpoint and its components over the blinded duration of the FIBRONEER-ILD trial.
Results for the components of the key secondary endpoint are exploratory. Values shown here are for descriptive purposes. a Blinded trial duration was up to 109 weeks.
bid = twice dailyFigure 5 Acute ILD Exacerbation, Hospitalization for Respiratory Cause or Death over the Blinded Duration of the Trial (FIBRONEER-ILD)a 
Survival
In the FIBRONEER-ILD trial population, the hazard ratios for all-cause mortality, assessed until the end of trial (up to 114 weeks), for JASCAYD 18 mg and 9 mg compared to placebo were 0.51 [95% CI: 0.34, 0.78] and 0.51 [95% CI: 0.34, 0.78], respectively. These results were not prespecified for multiplicity control.
Figure 6 shows the cumulative incidence of death in adults with PPF in the FIBRONEER-ILD trial for JASCAYD 18 mg and placebo.
bid = twice daily Figure 6 Cumulative Incidence of Death over the Duration of the Trial in Adults with PPF (FIBRONEER-ILD) 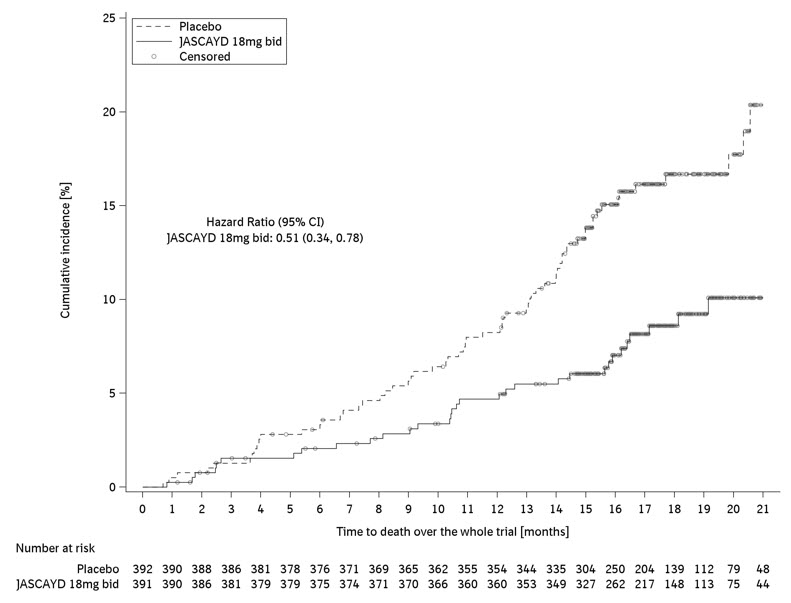
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
JASCAYD tablets are supplied as follows:
Tablet Strength Tablet Description Package Configuration NDC 9 mg light yellow, oval, biconvex, film-coated tablets debossed with the Boehringer Ingelheim company symbol on one side and "F9" on the other side Bottles of 60 with child-resistant closure 0597-2465-41 18 mg light red, oval, biconvex, film-coated tablets debossed with the Boehringer Ingelheim company symbol on one side and "F18" on the other side Bottles of 60 with child-resistant closure 0597-7313-21 -
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Pregnancy
Advise female patients to contact their healthcare provider if they become pregnant or suspect they may be pregnant during treatment with JASCAYD. Advise female patients of the potential risk of fetal loss [see Use in Specific Populations (8.1)].
Missed Dose
Inform patients that if they miss a dose of JASCAYD, they should take the next dose at the next scheduled time. Advise patients to not make up for a missed dose or exceed the recommended dosage of 18 mg twice daily [see Dosage and Administration (2.1)].
-
SPL UNCLASSIFIED SECTION
Distributed by:
Boehringer Ingelheim Pharmaceuticals, Inc.
Ridgefield, CT 06877 USALicensed from:
Boehringer Ingelheim International GmbH, Ingelheim, GermanyJASCAYD is a registered trademark of and used under license from Boehringer Ingelheim International GmbH.
Copyright © 2025 Boehringer Ingelheim International GmbH
ALL RIGHTS RESERVEDCOL11694CL182025
SPL14534D -
PATIENT PACKAGE INSERT
PATIENT INFORMATION
JASCAYD® (JASS-kayd)
(nerandomilast tablets)
for oral useThis Patient Information has been approved by the U.S. Food and Drug Administration. Revised: 12/2025 What is JASCAYD?
JASCAYD is a prescription medicine used:- to treat adults with a lung disease called idiopathic pulmonary fibrosis (IPF).
- to treat adults with a lung disease called progressive pulmonary fibrosis (PPF).
Before you take JASCAYD, tell your healthcare provider about all of your medical conditions, including if you: - have kidney problems.
- have liver problems.
- are pregnant or plan to become pregnant. JASCAYD may cause loss of your pregnancy (miscarriage). It is not known if JASCAYD can harm your unborn baby. Tell your healthcare provider right away if you become pregnant or think you are pregnant while taking JASCAYD.
- are breastfeeding or plan to breastfeed. It is not known if JASCAYD passes into your breastmilk. You and your healthcare provider should decide if you will take JASCAYD or breastfeed.
How should I take JASCAYD? - Take JASCAYD exactly as your healthcare provider tells you to take it.
- Your healthcare provider will tell you how much JASCAYD to take and when to take it.
- Take JASCAYD either with or without food.
- Swallow JASCAYD tablets whole.
- If you cannot swallow JASCAYD tablets whole:
- Place 3 to 4 ounces (about 100 mL) of non-carbonated, room temperature water in a glass. Do not use any other liquids.
- Place a JASCAYD tablet in the water, without crushing the tablet, and stir regularly for about 15 to 20 minutes until the tablet is in very small pieces (the tablet will not completely dissolve).
- Drink the JASCAYD and water mixture within 2 hours of mixing.
- If you do not drink the mixture right away, stir the mixture again before drinking.
- Rinse the glass with 3 to 4 ounces (about 100 mL) of water and drink to make sure that you have taken the full dose of JASCAYD.
- If you miss a dose of JASCAYD, take your next dose at your regular time. Do not take the missed dose.
- Do not take more than two 18 mg JASCAYD tablets in 1 day.
- If you take too much JASCAYD, call your healthcare provider or Poison Help line at 1-800-222-1222, or go to the nearest hospital emergency room right away.
What are the possible side effects of JASCAYD?
The most common side effects of JASCAYD include:- diarrhea
- COVID-19
- upper respiratory tract infection
- depression
- weight loss
- decreased appetite
- nausea
- fatigue
- headache
- vomiting
- back pain
- dizziness
These are not all of the possible side effects of JASCAYD. For more information, ask your healthcare provider or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. How should I store JASCAYD? - Store JASCAYD at room temperature between 68°F to 77°F (20°C to 25°C).
- Store JASCAYD in the original child-resistant container to protect it from light.
General information about the safe and effective use of JASCAYD.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use JASCAYD for any condition for which it was not prescribed. Do not give JASCAYD to other people, even if they have the same symptoms you have. It may harm them. You can ask your healthcare provider or pharmacist for information about JASCAYD that is written for health professionals.What are the ingredients in JASCAYD?
Active ingredient: nerandomilast
Inactive ingredients: croscarmellose sodium, ferric oxide red (18 mg tablets only), ferric oxide yellow (9 mg tablets only), ferrosoferric oxide (18 mg tablets only), hydroxypropyl cellulose, hypromellose, lactose monohydrate, magnesium stearate, mannitol, microcrystalline cellulose, polyethylene glycol, and talc. Each tablet contains less than 5 mg of sodium.Distributed by: Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT 06877 USA
Licensed from: Boehringer Ingelheim International GmbH, Ingelheim, Germany
JASCAYD is a registered trademark of and used under license from Boehringer Ingelheim International GmbH.
Copyright © 2025 Boehringer Ingelheim International GmbH ALL RIGHTS RESERVED
COL11694CL182025
For more information about JASCAYD, including current Prescribing Information and patient support, go to www.jascayd.com or call Boehringer Ingelheim Pharmaceuticals, Inc. at 1-800-542-6257, or scan the code to go to www.jascayd.com.

- PRINCIPAL DISPLAY PANEL - 9 mg Tablet Bottle Carton
- PRINCIPAL DISPLAY PANEL - 18 mg Tablet Bottle Carton
-
INGREDIENTS AND APPEARANCE
JASCAYD
nerandomilast tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0597-2465 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NERANDOMILAST (UNII: I5DGT51IB8) (NERANDOMILAST - UNII:I5DGT51IB8) NERANDOMILAST 9 mg Product Characteristics Color YELLOW (Light Yellow) Score no score Shape OVAL (biconvex) Size 10mm Flavor Imprint Code F9 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0597-2465-41 1 in 1 CARTON 10/07/2025 1 60 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA218764 10/07/2025 JASCAYD
nerandomilast tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0597-7313 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NERANDOMILAST (UNII: I5DGT51IB8) (NERANDOMILAST - UNII:I5DGT51IB8) NERANDOMILAST 18 mg Product Characteristics Color RED (Light Red) Score no score Shape OVAL (biconvex) Size 10mm Flavor Imprint Code F18 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0597-7313-21 1 in 1 CARTON 10/07/2025 1 60 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA218764 10/07/2025 Labeler - Boehringer Ingelheim Pharmaceuticals, Inc. (603175944)
Trademark Results [JASCAYD]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 JASCAYD 79376569 not registered Live/Pending |
Boehringer Ingelheim International GmbH 2023-06-21 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.

