GFA FIRST AID- benzalkonium chloride, benzalkonium chloride, lidocaine, bacitracin zinc,neomycin sulfate,polymyxin b sulfate, povidone-iodine, water, alcohol, aspirin, ibuprofen, acetaminophen, benzocaine, alcohol kit
GFA First Aid by
Drug Labeling and Warnings
GFA First Aid by is a Otc medication manufactured, distributed, or labeled by Genuine First Aid, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active Ingredient
- Purpose
- Use
- Warnings
- KEEP OUT OF REACH OF CHILDREN
- STOP USE
- DO NOT USE
- Directions
- Inactive Ingredients
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
- Active Ingredients
- Purpose
- INDICATIONS & USAGE
- Warnings
- DO NOT USE
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- Directions
- STORAGE AND HANDLING
- Inactive Ingredient
-
PRINCIPAL DISPLAY PANEL
Genuine Triple Antibiotic
First Aid Ointment
To Help Prevent Infection
Each Gram Contains:
Bacitracin Zinc 400 units
Neomycin Sulfate 5 mg
(equivalent to 3.5 mg
Neomycin base)
Polymyxin B Sulfate 5000 units
Net Wt. 0.5g ; (1/64 oz)
Manufactured in CHINA for
GENUINE FIRST AID.
Triple Antibiotic Ointment 10pcs
Net wt. 0.9g (1/32oz)
100
Triple Antibiotic
- Active Ingredient
- Purpose
- Uses
- Keep out of reach of children
- Caution
- Inactive Ingredients
- Directions
-
PRINCIPAL DISPLAY PANEL
Antiseptic
Povidone-Iodine
Prep Pads
medium
Saturated with a 10% Povidone-Iodine
Solution equavalent to 1% available iodine
GFA Production Xiamen Co., Ltd.
www.gfaproduction.com
GFA Production Xiamen Co., Ltd.
No. 20 Huli Industrial Park, Meixi Road,
Tong'an, Xiamen, Fujian, China 361100
Wellkang Ltd t/a Wellkang Tech Consulting
Suite B, 29 Harley Street
LONDON W1G 9QR, England, United Kingdom
- Active Ingredient
- Purpose
- Uses
- Warnings
- Directions
- Inactive Ingredients
- Keep out of reach of children
- PRINCIPAL DISPLAY PANEL
- Active Ingredients
- Purpose
- Uses
- Warnings
-
DO NOT USE
Do not use: In eyes, in large quantities, over raw blistered areas, or on deep puncture wounds, animal bites or serious burns, for more than one week
Do not use:
in the eyes or apply over large areas of the body.
longer than 1 week unless directed by a doctor.
in large quantities, particularly over raw surfaces or blistered areas.
Ask a doctor before use if you have deep puncture wounds, animal bites or serious burns.
When using this product, avoid contact with the eyes.
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- Directions
- STORAGE AND HANDLING
- Inactive Ingredients
- DESCRIPTION
- PRINCIPAL DISPLAY PANEL
- Active Ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Allergy alert: Ibuprofen may cause a severe allergic reaction, especially in people allergic to aspirin. Symptoms may include: shock, facial swelling, asthma (wheezing) rash, skin reddening, blisters, hives If an allergic reaction occurs, stop use and seek medical help right away.
Stomach bleeding warning: This product contains a nonsteroidal anti-inflammatory drug (NSAID), which may cause severe stomach bleeding. The chance is higher if you: are age 60 or older, have had stomach ulcers or bleeding problems, take a blood thinner (anticoagulant) or steroid drug, take other drugs containing NSAIDs (aspirin, ibuprofen, naproxen, or others), have 3 or more alcoholic drinks every day while using this product, take more or for a longer time than directed
- DO NOT USE
- ASK DOCTOR
- Ask a doctor before use if you are:
- When using this product
-
Stop use and ask a doctor if
you experience any of the following signs of stomach bleeding; feel faint; vomit blood; have bloody or black stools; have stomach pain that does get better; pain gets worse or lasts more than 10 days; fever gets worse or lasts more than 3 days; redness or swelling is present in the painful area; any new symptoms appear
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
Directions
do not use more than directed; the smallest effective dose should be used; do not take longer than 10 days, unless directed by a doctor.
Adults and Children (12 years and older): Take 1 tablet every 4 to 6 hours while symptoms persist. If pain or fever does not respond to 1 tablet, 2 tablets may be used. Do not exceed 6 tablets in 24 hours, unless directed by a doctor.
Children under 12 years: Do not give to children under 12 years of age.
- Other information
- Inactive Ingredients
- DESCRIPTION
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- Active Ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Liver warning: This product contains acetaminophen.
Severe liver damage may occur if: adult takes more than 12 tablets in 24 hours, which is the maximum daily amount; child takes more than 5 doses in 24 hours; taken with other drugs containing acetaminophen; adult has 3 or more alcoholic drinks every day while using this product
- Do not use
- ASK DOCTOR
- ASK DOCTOR/PHARMACIST
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- Inactive Ingredients
-
DOSAGE & ADMINISTRATION
Directions
Adults and Children Take 2 tablets every 4 to 6 hours as
12 years of age needed. Do not take more than 12 tablets
or older in 24 hours.
Children 6-11 years Take 1 tablet every 4 to 6 hours as
of age needed. Do not take more than 5
tablets in 24 hours.
Children under 6 Do not use this regular strength product.
years of age This will provide more than the
recommended dose (overdose) and could
cause serious health problems.
- STORAGE AND HANDLING
- GENERAL PRECAUTIONS
- DESCRIPTION
- PRINCIPAL DISPLAY PANEL
- Active Ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox of flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Allergy alert: Aspirin may cause a severe allergic reaction which may include: hives, skin reddening, facial swelling, rash, asthma (wheezing), blisters, shock, If an allergic reaction occurs, stop use and seek medical help right away.
Stomach bleeding warning: This contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you:
are age 60 or older; have had stomach ulcers or bleeding problems; take a blood thinner (anticoagulant) or steroid drug; take other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen, or others); have 3 or more alcoholic drinks every day while using this product; take more or for a longer time than directed
- Do not use
- ASK DOCTOR
- ASK DOCTOR/PHARMACIST
- WHEN USING
-
Stop use and ask a doctor if
Stop use and ask a doctor if
you experience any of the following signs of stomach bleeding:
feel faint; vomit blood; have bloody or black stools; have stomach
pain that does not get better; pain gets worse or lasts more than 10 days; fever gets worse or lasts more than 3 days; you have difficulty swallowing; if ringing in the ears or loss of hearing occurs; redness or swelling is present in the painful areas; any new symptoms appear
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- Directions
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
- Inactive Ingredients
- DESCRIPTION
- PRINCIPAL DISPLAY PANEL
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- STORAGE AND HANDLING
- WARNINGS
- DOSAGE & ADMINISTRATION
- PURPOSE
- DESCRIPTION
- PRINCIPAL DISPLAY PANEL
- Active Ingredient
- Purpose
- Use
- Warnings
- STORAGE AND HANDLING
- DO NOT USE
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Inactive Ingredient
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
- Active Ingredient
- Purpose
- Uses
- Directions
- Warnings
- Keep out of reach of children
- Inactive Ingredients
- STORAGE AND HANDLING
- DO NOT USE
- DESCRIPTION
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
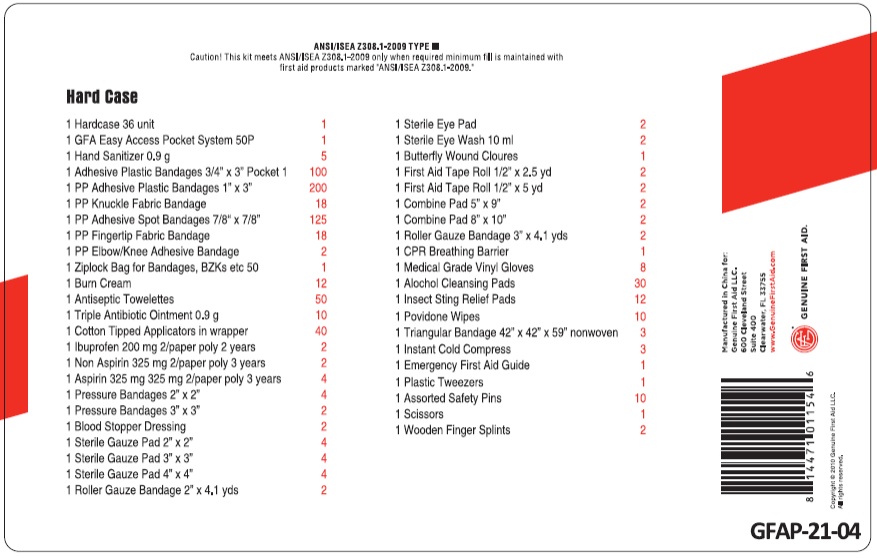
GFA FIRST AID 75 PERSON METAL CASE
benzalkonium chloride, benzalkonium chloride, lidocaine, bacitracin zinc,neomycin sulfate,polymyxin b sulfate, povidone-iodine, water, alcohol, aspirin, ibuprofen, acetaminophen, benzocaine, alcohol kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 52124-1154 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52124-1154-1 1 in 1 KIT Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 50 PACKAGE 40.0 mL Part 2 12 PACKET 10.8 g Part 3 10 TUBE 5.0 g Part 4 10 PACKET 5 mL Part 5 2 BOTTLE 20 mL Part 6 5 PACKET 4.5 g Part 7 30 PACKAGE 15 mL Part 8 12 PACKAGE 6 mL Part 9 2 PACKET 4 Part 10 2 PACKAGE 4 Part 11 4 PACKAGE 8 Part 1 of 11 ANTISEPTIC TOWELETTE
benzalkonium chloride swabProduct Information Item Code (Source) NDC: 52124-0001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.4 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52124-0001-1 0.8 mL in 1 PACKAGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 05/03/2011 Part 2 of 11 GENUINE FIRST AID BURN ANTISEPTIC PAIN RELIEF WITH ALOE
benzalkonium chloride, lidocaine creamProduct Information Item Code (Source) NDC: 52124-0004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 g LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 0.5 g in 100 g Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) CETYL ALCOHOL (UNII: 936JST6JCN) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) METHYLPARABEN (UNII: A2I8C7HI9T) MINERAL OIL (UNII: T5L8T28FGP) POLYETHYLENE GLYCOL (UNII: 3WJQ0SDW1A) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) STEARIC ACID (UNII: 4ELV7Z65AP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52124-0004-1 0.9 g in 1 PACKET Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333A 04/13/2011 Part 3 of 11 GENUINE TRIPLE ANTIBIOTIC
bacitracin zinc,neomycin sulfate,polymyxin b sulfate ointmentProduct Information Item Code (Source) NDC: 52124-0003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BACITRACIN ZINC (UNII: 89Y4M234ES) (BACITRACIN - UNII:58H6RWO52I) BACITRACIN ZINC 400 [iU] in 1 g NEOMYCIN SULFATE (UNII: 057Y626693) (NEOMYCIN - UNII:I16QD7X297) NEOMYCIN SULFATE 5 mg in 1 g POLYMYXIN B SULFATE (UNII: 19371312D4) (POLYMYXIN B - UNII:J2VZ07J96K) POLYMYXIN B SULFATE 5000 [iU] in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52124-0003-1 0.5 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333B 04/13/2011 Part 4 of 11 POVIDONE-IODINE PREP
povidone-iodine swabProduct Information Item Code (Source) NDC: 52124-2901 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POVIDONE-IODINE (UNII: 85H0HZU99M) (IODINE - UNII:9679TC07X4) IODINE 1 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52124-2901-0 0.5 mL in 1 PACKET Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 05/03/2011 Part 5 of 11 STERILE ISOTONIC BUFFERED GENUINE EYEWASH
water liquidProduct Information Item Code (Source) NDC: 52124-0005 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 98.16 mL in 100 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM PHOSPHATE, MONOBASIC (UNII: 3980JIH2SW) SODIUM PHOSPHATE, DIBASIC (UNII: GR686LBA74) EDETATE DISODIUM (UNII: 7FLD91C86K) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52124-0005-1 10 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part349 05/04/2011 Part 6 of 11 GENUINE HAND SANITIZER
alcohol gelProduct Information Item Code (Source) NDC: 52124-2906 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 62 g in 100 g Inactive Ingredients Ingredient Name Strength PROPYLENE GLYCOL (UNII: 6DC9Q167V3) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52124-2906-1 0.9 g in 1 PACKET Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 03/28/2011 Part 7 of 11 ALCOHOL CLEANSING PAD
isopropyl alcohol liquidProduct Information Item Code (Source) NDC: 52124-0002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52124-0002-1 0.5 mL in 1 PACKAGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 04/23/2010 Part 8 of 11 INSECT STING RELIEF PAD
benzocaine,alcohol swabProduct Information Item Code (Source) NDC: 52124-0008 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 6 mL in 100 mL ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 60 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52124-0008-1 0.5 mL in 1 PACKAGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part348 04/23/2011 Part 9 of 11 IBUPROFEN
ibuprofen tabletProduct Information Item Code (Source) NDC: 52124-0009 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IBUPROFEN (UNII: WK2XYI10QM) (IBUPROFEN - UNII:WK2XYI10QM) IBUPROFEN 200 mg Inactive Ingredients Ingredient Name Strength POWDERED CELLULOSE (UNII: SMD1X3XO9M) STARCH, CORN (UNII: O8232NY3SJ) HYPROMELLOSES (UNII: 3NXW29V3WO) LACTOSE (UNII: J2B2A4N98G) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYDEXTROSE (UNII: VH2XOU12IE) POLYETHYLENE GLYCOL (UNII: 3WJQ0SDW1A) POVIDONE (UNII: FZ989GH94E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STEARIC ACID (UNII: 4ELV7Z65AP) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) Product Characteristics Color white (White) Score no score Shape ROUND Size 10mm Flavor Imprint Code 44;352 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52124-0009-1 2 in 1 PACKET Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA075010 02/17/2010 Part 10 of 11 NON-ASPIRIN
acetaminophen tabletProduct Information Item Code (Source) NDC: 52124-0010 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 325 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) POLYETHYLENE GLYCOL (UNII: 3WJQ0SDW1A) STEARIC ACID (UNII: 4ELV7Z65AP) POVIDONE (UNII: FZ989GH94E) Product Characteristics Color white (White) Score no score Shape ROUND Size 11mm Flavor Imprint Code AZ;234 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52124-0010-1 2 in 1 PACKAGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part343 04/23/2010 Part 11 of 11 ASPIRIN
aspirin tabletProduct Information Item Code (Source) NDC: 52124-0011 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASPIRIN (UNII: R16CO5Y76E) (ASPIRIN - UNII:R16CO5Y76E) ASPIRIN 325 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSES (UNII: 3NXW29V3WO) POLYETHYLENE GLYCOL (UNII: 3WJQ0SDW1A) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color white (White) Score no score Shape ROUND Size 11mm Flavor Imprint Code 44;157;ASPIRIN Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52124-0011-1 2 in 1 PACKAGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part343 04/23/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333E 05/03/2011 Labeler - Genuine First Aid, LLC (619609857)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.