Diazepam by Meridian Medical Technologies LLC DIAZEPAM injection
Diazepam by
Drug Labeling and Warnings
Diazepam by is a Prescription medication manufactured, distributed, or labeled by Meridian Medical Technologies LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
BOXED WARNING
(What is this?)
WARNING: RISKS FROM CONCOMITANT USE WITH OPIOIDS
Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death [see WARNINGS and PRECAUTIONS].
- Reserve concomitant prescribing of these drugs for use in patients for whom alternative treatment options are inadequate.
- Limit dosages and durations to the minimum required.
- Follow patients for signs and symptoms of respiratory depression and sedation.
-
DESCRIPTION
Diazepam injection is a sterile solution packaged within a device that delivers its entire 2 mL contents automatically upon activation. Each mL contains 5 mg diazepam compounded with 40% propylene glycol, 10% ethyl alcohol, 5% sodium benzoate and benzoic acid as buffers, and 1.5% benzyl alcohol as preservative.
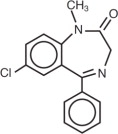
Diazepam is a benzodiazepine derivative. Chemically, diazepam is 7-chloro-1,3- dihydro-1-methyl-5-phenyl-2H-1, 4-benzodiazepin-2-one. It is a colorless crystalline compound, insoluble in water and has a molecular weight of 284.74. Its structural formula is as follows:
-
CLINICAL PHARMACOLOGY
In animals, diazepam appears to act on parts of the limbic system, the thalamus and hypothalamus, and induces calming effects. Diazepam, unlike chlorpromazine and reserpine, has no demonstrable peripheral autonomic blocking action, nor does it produce extrapyramidal side effects. However, animals treated with diazepam do have a transient ataxia at higher doses. Diazepam was found to have transient cardiovascular depressor effects in dogs. Long-term experiments in rats revealed no disturbances of endocrine function. Injections into animals have produced localized irritation of tissue surrounding injection sites and some thickening of veins after intravenous use.
Pharmacokinetics (autoinjector):
A study performed in 24 healthy male subjects comparing the I.M. Injection of 10 mg of diazepam in the mid-anterior/lateral thigh by the autoinjector versus 10 mg I.M. by a syringe (operated manually) indicates that the mean percent availability of the drug from the autoinjector is 100% of that obtained from the syringe.
In addition, the mean Cmax value from the autoinjector was 314 ng/mL (c.v. = 18.7%, range 185 to 439 ng/mL) and 48.6 ng/mL (c.v.= 19.8%, range 29.4 to 69.7 ng/mL) for diazepam and desmethyldiazepam, respectively, while the syringe gave corresponding values of 287 ng/mL (c.v. = 18.9%, range 174 to 378 ng/mL) and 47.2 ng/mL (c.v. =
19.4%, range 33.1 to 61.2 ng/mL) for diazepam and desmethyldiazepam, respectively.
The corresponding mean Tmax values were 1.47 hours (c.v. = 69.9%, range 0.8 to 6 hours) and 61.0 hours (c.v. = 56.8%, range 24 to 144 hours) for diazepam and desmethyldiazepam for the autoinjector whereas the syringe gave values of 1.31 hours (c.v. = 32%, range 0.7 to 2.0 hours) and 54.5 hours (c.v. = 47.3%, range 12 to 96 hours) for diazepam and desmethyldiazepam, respectively.
-
INDICATIONS
Diazepam is indicated for the management of anxiety disorders for the short-term relief of the symptoms of anxiety. Anxiety or tension associated with the stress of everyday life usually does not require treatment with an anxiolytic.
- Note: Because the autoinjector provides a minimum dose of 10 mg diazepam, it should not be used to treat individuals with mild and moderate degrees of anxiety and anxiety related disorders that would ordinarily be managed with intramuscular doses of less than 10 mg.
In acute alcohol withdrawal, diazepam may be useful in the symptomatic relief of acute agitation, tremor, impending or acute delirium tremens and hallucinosis.
As an adjunct prior to endoscopic procedures if apprehension, anxiety or acute stress reactions are present, and to diminish the patient's recall of the procedures (See WARNINGS).
Diazepam is a useful adjunct for the relief of skeletal muscle spasm due to reflex spasm to local pathology (such as inflammation of the muscles or joints, or secondary to trauma), spasticity caused by upper motor neuron disorders (such as cerebral palsy and paraplegia); athetosis; stiff man syndrome; and tetanus.
Diazepam injection is a useful adjunct in status epilepticus and severe recurrent convulsive seizures.
Diazepam is useful premedication for relief of anxiety and tension in patients who are to undergo surgical procedures.
- CONTRAINDICATIONS
-
WARNINGS
Risks from Concomitant Use With Opioids
Concomitant use of benzodiazepines, including diazepam, and opioids may result in profound sedation, respiratory depression, coma, and death. Because of these risks, reserve concomitant prescribing of benzodiazepines and opioids for use in patients for whom alternative treatment options are inadequate.
Observational studies have demonstrated that concomitant use of opioid analgesics and benzodiazepines increases the risk of drug-related mortality compared to use of opioids alone. If a decision is made to prescribe diazepam concomitantly with opioids, prescribe the lowest effective dosages and minimum durations of concomitant use, and follow patients closely for signs and symptoms of respiratory depression and sedation. Advise both patients and caregivers about the risks of respiratory depression and sedation when diazepam is used with opioids [see PRECAUTIONS].
Diazepam Autoinjector is to be administered only by the intramuscular (I.M.) route.
Respiratory and Central Nervous System Depression
Extreme care must be used in administering injectable diazepam to the elderly, to very ill patients and to those with limited pulmonary reserve because of the possibility that apnea and/or cardiac arrest may occur. Concomitant use of barbiturates, alcohol, or other central nervous system depressants increases depression with increased risk of apnea. Resuscitative equipment including that necessary to support respiration should be readily available.
When diazepam is used with a narcotic analgesic, the dosage of the narcotic should be reduced by at least one-third and administered in small increments. In some cases the use of a narcotic may not be necessary [see PRECAUTIONS].
Diazepam injection should not be administered to patients in shock, coma, or in acute alcoholic intoxication with depression of vital signs. As is true of most CNS-acting drugs, patients receiving diazepam should be cautioned against engaging in hazardous occupations requiring complete mental alertness, such as operating machinery or driving a motor vehicle.
Use in Patients with Petit Mal Status
Tonic status epilepticus has been precipitated in patients treated with I.V. diazepam for petit mal status or petit mal variant status.
Usage in Pregnancy
An increased risk of congenital malformations associated with the use of minor tranquilizers (diazepam, meprobamate and chlordiazepoxide) during the first trimester of pregnancy has been suggested in several studies. Because use of these drugs is rarely a matter of urgency, their use during this period should almost always be avoided. The possibility that a woman of childbearing potential may be pregnant at the time of institution of therapy should be considered. Patients should be advised that if they become pregnant during therapy or intend to become pregnant they should communicate with their physicians about the desirability of discontinuing the drug.
In humans, measurable amounts of diazepam were found in maternal and cord blood, indicating placental transfer of the drug. Until additional information is available, diazepam injection is not recommended for obstetrical use.
Pediatric Use
Efficacy and safety of parenteral diazepam has not been established in the neonate (30 days or less of age).
Prolonged central nervous system depression has been observed in neonates, apparently due to inability to biotransform diazepam into inactive metabolites.
Withdrawal Symptoms
Withdrawal symptoms of the barbiturate type have occurred after the discontinuation of benzodiazepines (see DRUG ABUSE AND DEPENDENCE section).
-
PRECAUTIONS
Drug Interactions:
Effect of Concomitant Use of Benzodiazepines and Opioids
The concomitant use of benzodiazepines and opioids increases the risk of respiratory depression because of actions at different receptor sites in the CNS that control respiration. Benzodiazepines interact at GABAA sites, and opioids interact primarily at mu receptors. When benzodiazepines and opioids are combined, the potential for benzodiazepines to significantly worsen opioid-related respiratory depression exists. Limit dosage and duration of concomitant use of benzodiazepines and opioids, and follow patients closely for respiratory depression and sedation.
Concomitant Use with Other Psychotropic Agents or Anticonvulsant Drugs
If diazepam is to be combined with other psychotropic agents or anticonvulsant drugs, careful consideration should be given to the pharmacology of the agents to be employed
- particularly with known compounds which may potentiate the action of diazepam, such as phenothiazines, narcotics, barbiturates, MAO inhibitors and other antidepressants. In highly anxious patients with evidence of accompanying depression, particularly those who may have suicidal tendencies, protective measures may be necessary. The usual precautions in treating patients with impaired hepatic function should be observed. Metabolites of diazepam are excreted by the kidney; to avoid their excess accumulation, caution should be exercised in the administration to patients with compromised kidney function.
Administration Considerations:
Long Term Control of Seizures
Although seizures may be brought under control promptly with a single 10 mg intramuscular dose, a significant proportion of patients may experience a return to seizure activity. Consequently, it may become necessary to re-administer the drug. The cumulative maximum dose of diazepam should not exceed 30 mg; the interval between doses should be no less than 10 minutes. Diazepam is not recommended for maintenance, and once seizures are brought under control, consideration should be given to the administration of agents useful in longer term control of seizures.
Since an increase in cough reflex and laryngospasm may occur with peroral endoscopic procedures, the use of a topical anesthetic agent and the availability of necessary countermeasures are recommended.
-
ADVERSE REACTIONS
Side effects most commonly reported were drowsiness, fatigue and ataxia; venous thrombosis and phlebitis at the site of injection. Other adverse reactions less frequently reported include: CNS: confusion, depression, dysarthria, headache, hypoactivity, slurred speech, syncope, tremor, vertigo. G.I.: constipation, nausea. G.U.: incontinence, changes in libido, urinary retention. Cardiovascular: bradycardia, cardiovascular collapse, hypotension. EENT: blurred vision, diplopia, nystagmus. Skin: urticaria, skin rash. Other: hiccups, changes in salivation, neutropenia, jaundice. Paradoxical reactions such as acute hyperexcited states, anxiety, hallucinations, increased muscle spasticity, insomnia, rage, sleep disturbances and stimulation have been reported; should these occur, use of the drug should be discontinued.
Minor changes in EEG patterns, usually low-voltage fast activity, have been observed in patients during and after diazepam therapy and are of no known significance.
In peroral endoscopic procedures, coughing, depressed respiration, dyspnea, hyperventilation, laryngospasm and pain in throat or chest have been reported.
Because of isolated reports of neutropenia and jaundice, periodic blood counts and liver function tests are advisable during long-term therapy.
-
DRUG ABUSE AND DEPENDENCE
Diazepam injection is classified by the Drug Enforcement Administration as a schedule IV controlled substance.
Withdrawal symptoms, similar in character to those noted with barbiturates and alcohol (convulsions, tremor, abdominal and muscle cramps, vomiting and sweating), have occurred following abrupt discontinuance of diazepam. The more severe withdrawal symptoms have usually been limited to those patients who received excessive doses over an extended period of time.
Generally milder withdrawal symptoms (e.g. dysphoria and insomnia) have been reported following abrupt discontinuance of benzodiazepines taken continuously at therapeutic levels for several months. Consequently, after extended therapy, abrupt discontinuation should generally be avoided and a gradual dosage tapering schedule followed. Addiction-prone individuals (such as drug addicts or alcoholics) should be under careful surveillance when receiving diazepam or other psychotropic agents because of the predisposition of such patients to habituation and dependence.
-
DOSAGE AND ADMINISTRATION (INTRAMUSCULAR ONLY)
Administration of the Diazepam Autoinjector
- Pull off gray safety cap
- Place Black end on mid outer thigh
- Push hard until injector functions
- Withdraw after 10 seconds
Dosage:
Caution: Because the autoinjector automatically delivers a fixed dose of 10 mg of diazepam, it cannot be used in situations requiring lower total doses or those in which small incremental increases of diazepam are required.
The usual recommended dose in older children and adults ranges from 10 mg to 20 mg I.M. depending on the indication and its severity. The cumulative total dose and individual maximum dose for intramuscular administration will vary with the specific indication (See dosage for specific indications).
Intramuscular: When instructions are followed properly the Diazepam Autoinjector injects deeply into the muscle.
Intravenous Use: The Diazepam Autoinjector is not designed or intended for intravenous use.
Severe Anxiety Disorders and Symptoms of Anxiety:
Usual Adult Dosage: 10 mg, I. M. Repeat in 3 to 4 hours, if necessary.
Acute Alcohol Withdrawal: As an aid in symptomatic relief of acute agitation, tremor, impending or acute delirium tremens and hallucinosis.
Usual Adult Dosage: 10 mg, I. M. initially, then 10 mg in 3 to 4 hours, if necessary.
Endoscopic procedures: Adjunctively, if apprehension, anxiety or acute stress reactions are present prior to endoscopic procedures. Dosage of narcotics should be reduced by at least a third and in some cases may be omitted. See Precautions for peroral procedures.
Usual Adult Dosage: 10 mg I. M., approximately 30 minutes prior to the procedure.
Muscle Spasm: Associated with local pathology, cerebral palsy, athetosis, stiff-man syndrome or tetanus.
Usual Adult Dosage: 10 mg, I. M., initially, then 10 mg in 3 to 4 hours, if necessary. For tetanus, larger doses may be required.
Status Epilepticus and Severe Recurrent Convulsive Seizures: In the convulsing patient, the I.V. route is preferred. However, if conditions preclude intravenous administration, the I.M. route may be used.
Usual Adult Dosage: 10 mg, initially. This injection may be repeated if necessary at
10 to 15 minute intervals up to a maximum dose of 30 mg. If necessary, therapy with diazepam may be repeated in 2 to 4 hours; however, residual active metabolites may persist, and re-administration should be made with this consideration.
Extreme caution must be exercised with individuals with chronic lung disease or unstable cardiovascular status.
Preoperative Medication: To relieve anxiety and tension. (if atropine, scopolamine or other premedications are desired, they must be administered in separate syringes).
Usual Adult Dosage: 10 mg, I.M. (preferred route), before surgery.
General:
Once the acute symptomatology has been properly controlled with diazepam injection, the patient may be placed on oral therapy with diazepam if further treatment is required.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever the solution and container permit.
Management of Overdosage:
Manifestations of diazepam overdosage include somnolence, confusion, coma and diminished reflexes. Respiration, pulse and blood pressure should be monitored as in all cases of drug overdosage, although, in general, these effects have been minimal. General supportive measures should be employed, along with intravenous fluids, and an adequate airway maintained. Hypotension may be combated by the use of norepinephrine or metaraminol. Dialysis is of limited value.
Flumazenil, a specific benzodiazepine receptor antagonist, is indicated for the complete or partial reversal of the sedative effects of benzodiazapine and may be used in situations when an overdose with a benzodiazepine is known or suspected. Prior to the administration of flumazenil, necessary measures should be instituted to secure airways, ventilation, and intravenous access. Flumazenil is intended as an adjunct to, not as a substitute for, proper management of benzodiazepine overdose. Patients treated with flumazenil should be monitored for resedation, respiratory depression, and other residual benzodiazepine effects for an appropriate period after treatment. The prescriber should be aware of a risk of seizure in association with flumazenil treatment, particularly in long term benzodiazepine users and in cyclic antidepressant overdosage. The complete flumazenil package insert including CONTRAINDICATIONS, WARNINGS, and PRECAUTIONS should be consulted prior to use.
- HOW SUPPLIED:
-
ANIMAL PHARMACOLOGY:
Oral LD50 of diazepam is 720 mg/kg in mice and 1240 mg/kg in rats. Intraperitoneal administration of 400 mg/kg to a monkey resulted in death on the sixth day.
Reproduction Studies: A series of rat reproduction studies was performed with diazepam in oral doses of 1, 10, 80 and 100 mg/kg given for periods ranging from 60-228 days prior to mating. At 100 mg/kg there was a decrease in the number of pregnancies and surviving offspring in these rats. These effects may be attributable to prolonged sedative activity, resulting in lack of interest in mating and lessened maternal nursing and care of the young. Neonatal survival of rats at doses lower than 100 mg/kg was within normal limits. Several neonates, both controls and experimentals, in these rat reproduction studies showed skeletal or other defects. Further studies in rats at doses up to and including 80 mg/kg/day did not reveal significant teratological effects on the offspring. Rabbits were maintained on doses of 1, 2, 5 and 8 mg/kg from day 6 through day 18 of gestation. No adverse effects on reproduction and no teratological changes were noted.
-
STORAGE:
Store at 25°C (77°F); Excursions permitted to 15-30°C (59-86°F). [See USP Controlled Room Temperature]. Do not refrigerate.
Office of the Surgeon General
U.S. Army Medical Research and Development Command (MCMR-RCQ-HR)
Fort Detrick
Frederick, Maryland 21702-5012Manufactured by: Revision: December 2016
Meridian Medical Technologies, Inc.
Columbia, MD 21046
A Pfizer company 0001975 -
Principal Display Panel - 2 mL Injector Label
MERIDIAN
MEDICAL TECHNOLOGIESTM
Columbia, MD 21046
A subsidiary of King
Pharmaceuticals®, Inc.
AUTOINJECTOR
FOR BUDDY USEStore at 25°C (77°F); Excursions permitted
to 15-30°C (59-86°F).[See USP Controlled
Room Temperature]. Do not refrigerate.AUTOINJECTOR
FOR BUDDY USERx Only
1. Pull off gray safety cap
2. Place black end onmid outerthigh and
3. Push hard until injector functions
4. Withdraw after 10 seconds
MERIDIAN
MEDICAL TECHNOLOGIESTM
3 NDC 11704 60001 5
NSN 6505-01-274-0951 C-IV
DIAZEPAM INJECTION, USP, 10 mg AUTOMATIC
-
INGREDIENTS AND APPEARANCE
DIAZEPAM
diazepam injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 11704-600 Route of Administration INTRAMUSCULAR DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength diazepam (UNII: Q3JTX2Q7TU) (diazepam - UNII:Q3JTX2Q7TU) diazepam 10 mg in 2 mL Inactive Ingredients Ingredient Name Strength Sodium Benzoate (UNII: OJ245FE5EU) Benzoic Acid (UNII: 8SKN0B0MIM) Propylene Glycol (UNII: 6DC9Q167V3) Alcohol (UNII: 3K9958V90M) Benzyl Alcohol (UNII: LKG8494WBH) Water (UNII: 059QF0KO0R) Nitrogen (UNII: N762921K75) Sodium Hydroxide (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 11704-600-01 1 in 1 POUCH 12/05/1990 1 2 mL in 1 SYRINGE, GLASS; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020124 12/05/1990 Labeler - Meridian Medical Technologies, Inc. (167671341) Establishment Name Address ID/FEI Business Operations Meridian Medical Technologies, Inc. 038889234 MANUFACTURE(11704-600) , ANALYSIS(11704-600) Establishment Name Address ID/FEI Business Operations Meridian Medical Technologies, Inc. 078808315 MANUFACTURE(11704-600) , LABEL(11704-600) , PACK(11704-600) Establishment Name Address ID/FEI Business Operations Meridian Medical Technologies, Inc. 167671341 MANUFACTURE(11704-600) , LABEL(11704-600) , PACK(11704-600) , ANALYSIS(11704-600)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.