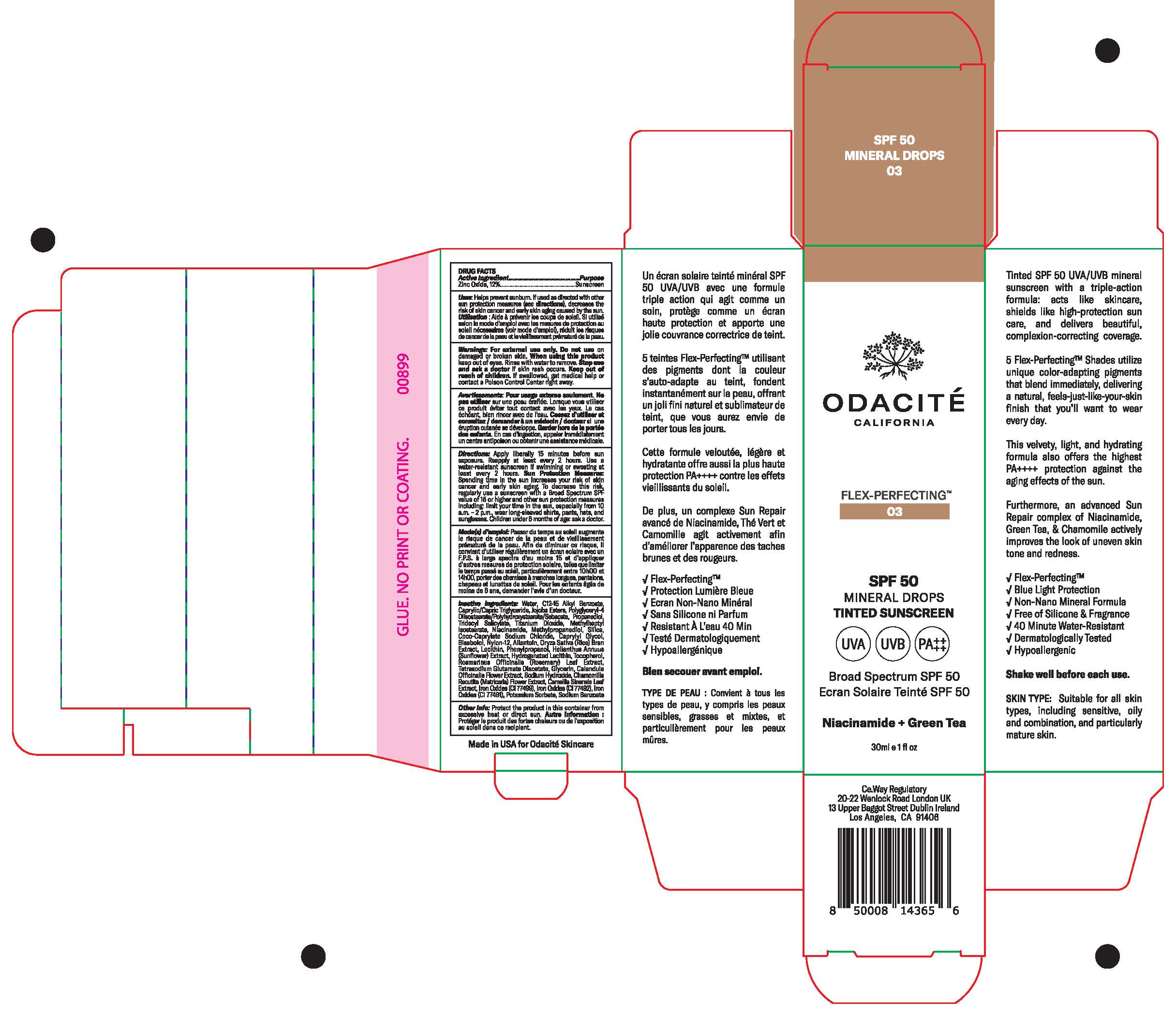
Odacite Flex Perfecting by Odacite Beauty / Nanophase Technologies Corporation Odacite Flex Perfecting
Odacite Flex Perfecting by
Drug Labeling and Warnings
Odacite Flex Perfecting by is a Otc medication manufactured, distributed, or labeled by Odacite Beauty, Nanophase Technologies Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ODACITE FLEX PERFECTING- zinc oxide lotion
Odacite Beauty
----------
Odacite Flex Perfecting
| ODACITE FLEX PERFECTING
zinc oxide lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Odacite Beauty (079682742) |
| Registrant - Nanophase Technologies Corporation (623502044) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Nanophase Technologies Corporation | 050383046 | api manufacture(82088-167) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Nanophase Technologies Corporation | 118812921 | manufacture(82088-167) , pack(82088-167) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Nanophase Technologies Corporation | 623502044 | api manufacture(82088-167) , manufacture(82088-167) | |
Revised: 10/2024
Document Id: 254c9178-e695-8324-e063-6294a90aa167
Set id: fa3e58ed-b41f-642b-e053-6294a90aa0ea
Version: 2
Effective Time: 20241025
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.