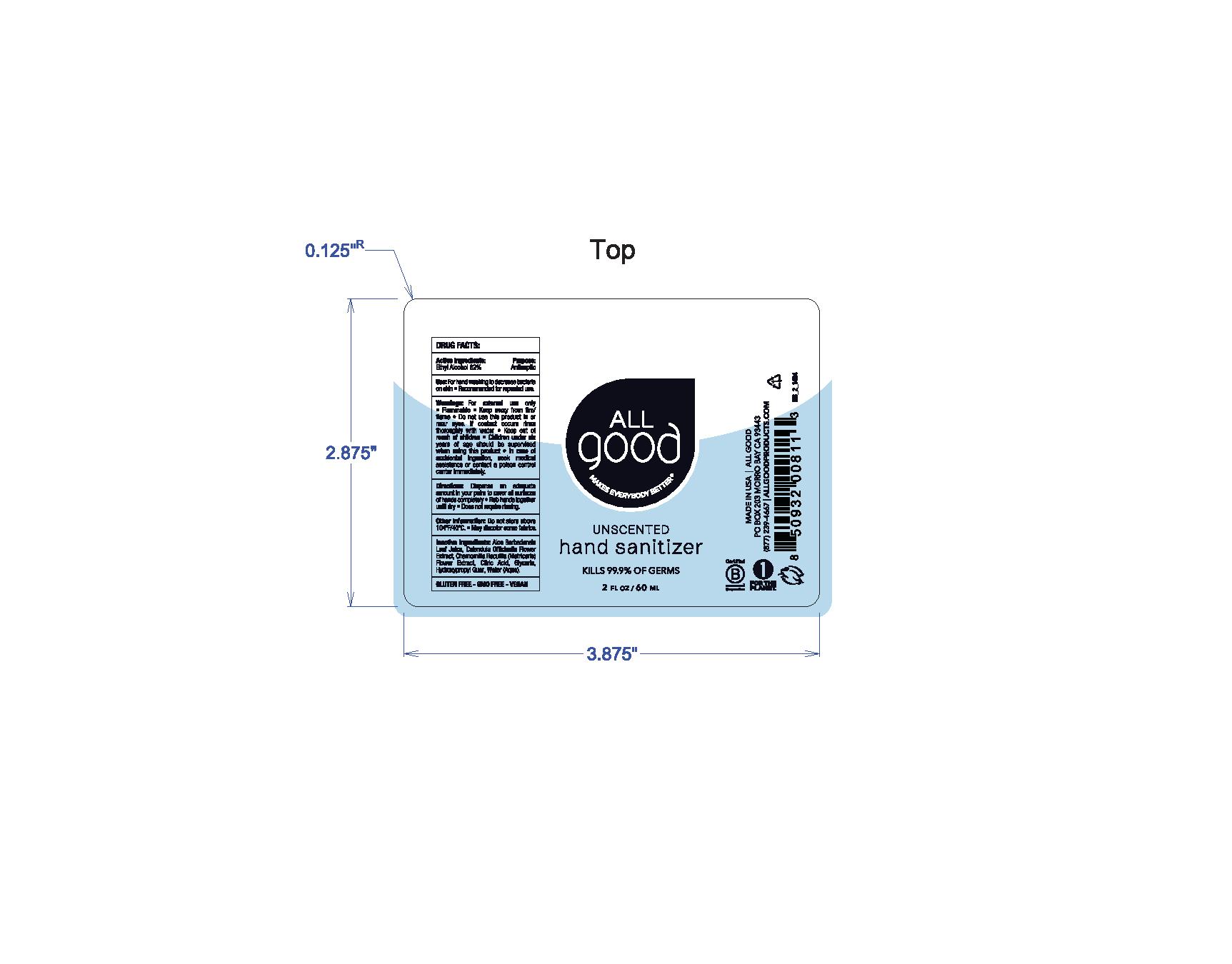
All Good - Unscented Hand Sanitizer Gel (01-EC-006)
All Good Unscented Hand Sanitizer by
Drug Labeling and Warnings
All Good Unscented Hand Sanitizer by is a Otc medication manufactured, distributed, or labeled by Elemental Herbs, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ALL GOOD UNSCENTED HAND SANITIZER- ethyl alcohol gel
Elemental Herbs, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
All Good - Unscented Hand Sanitizer Gel (01-EC-006)
Warning
For external use only
Flammable
Keep away from fire/flame.
Do not use this product in or near eyes. If contact occurs rinse thoroughly with water.
Directions
Dispense an adequate amount in your palm and cover all surface of hands completely
Rub hand together until dry
does not require rinsing
| ALL GOOD UNSCENTED HAND SANITIZER
ethyl alcohol gel |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Elemental Herbs, Inc. (132776357) |
Revised: 4/2023
Document Id: fa6c43f8-b1cb-03d5-e053-6294a90a220a
Set id: fa6c1227-3818-7b2a-e053-6294a90a79b2
Version: 1
Effective Time: 20230428
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.