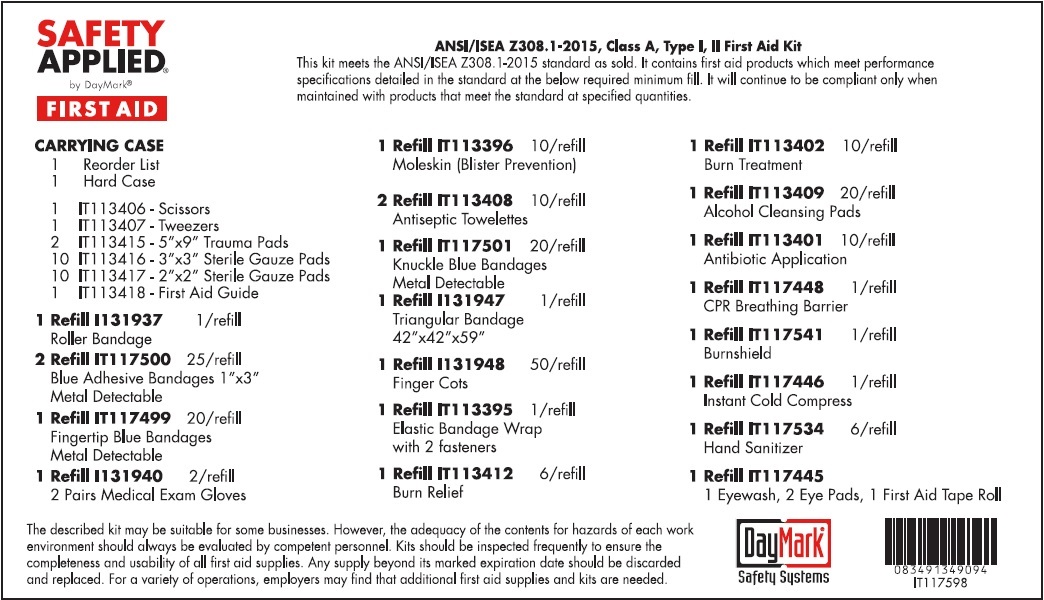
LARGE ANSI FIRST AID KIT- water, benzalkonium chloride, isopropyl alcohol, bacitracin zinc, neomycin sulfate, polymyxin b sulfate, lidocaine hydrochloride kit
Large ANSI First Aid Kit by
Drug Labeling and Warnings
Large ANSI First Aid Kit by is a Otc medication manufactured, distributed, or labeled by CMC Group, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Drug Facts - Eye Wash
- Active ingredient
- Use
-
Warnings
For external use only.
When using this product
to avoid contamination, do not touch tip of container to any surface do not reuse once opened, discard obtain immediate medical treatment for all open wounds in or near the eyes
- Directions
- Other information
- Inactive ingredients
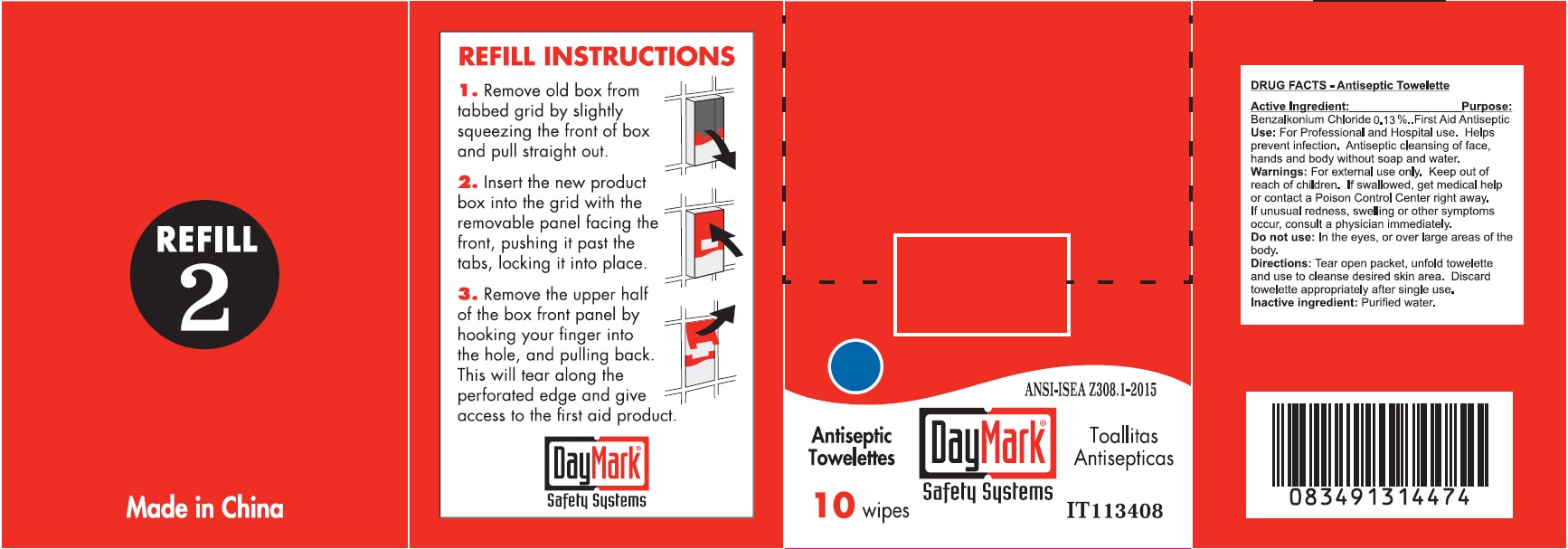
- Drug Facts - Antiseptic Towelettes
- Active Ingredient:
- Use:
- Warnings:
- Directions:
- Inactive ingredient:
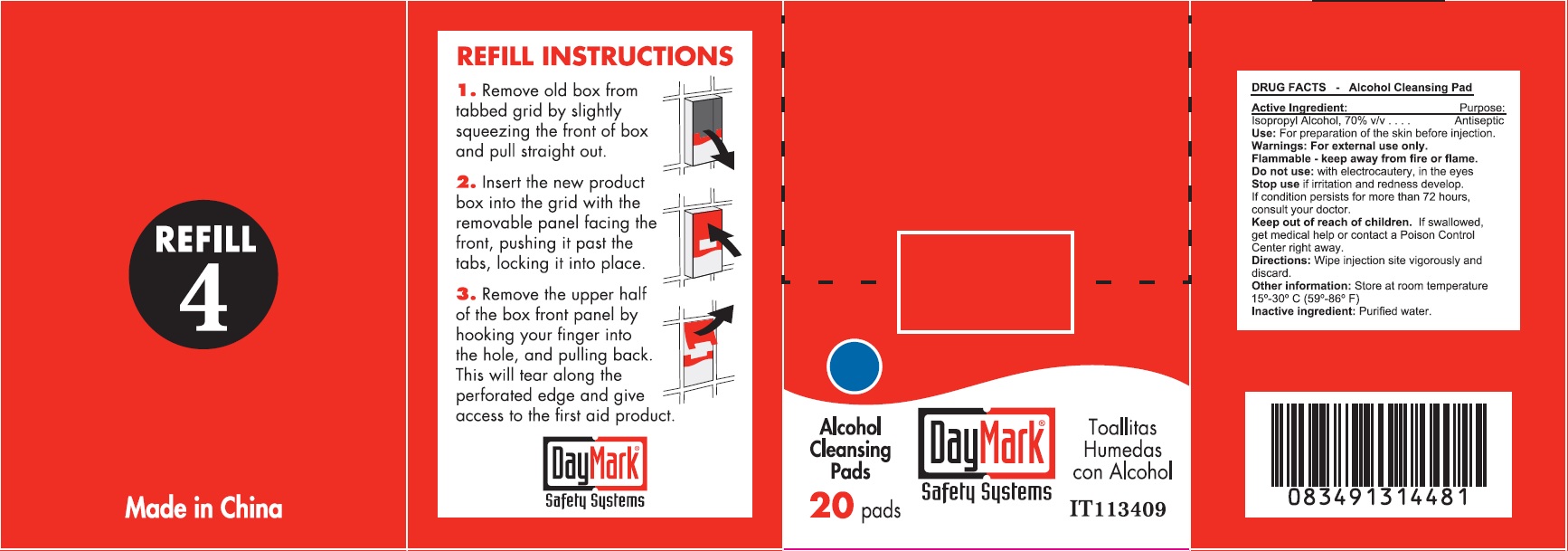
- DRUG FACTS - Alcohol Cleansing Pads
- Active Ingredient:
- Use:
- Warnings:
- Directions:
- Other information:
- Inactive ingredient:
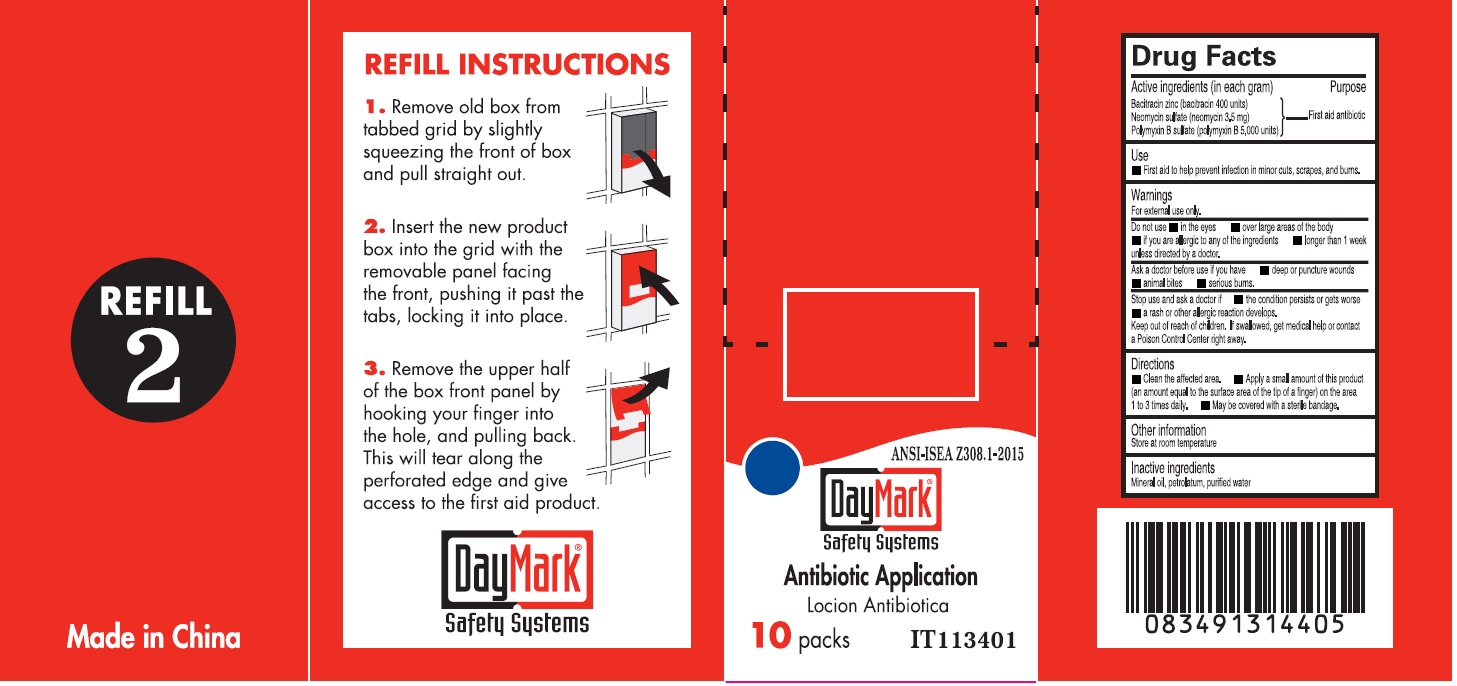
- Drug Facts - Antibiotic Application
- Active ingredients (in each gram)
- Use
- Warnings
- Directions
- Inactive ingredients
- Drug Facts - Burn Treatment
- Active ingredients
- Uses
-
Warnings
For external use only.
Do not use
in the eyes over large areas of the body in large quantities over raw surfaces or blistered areas longer than 1 week unless directed by a doctor
- Directions
- Other information
- Inactive ingredients
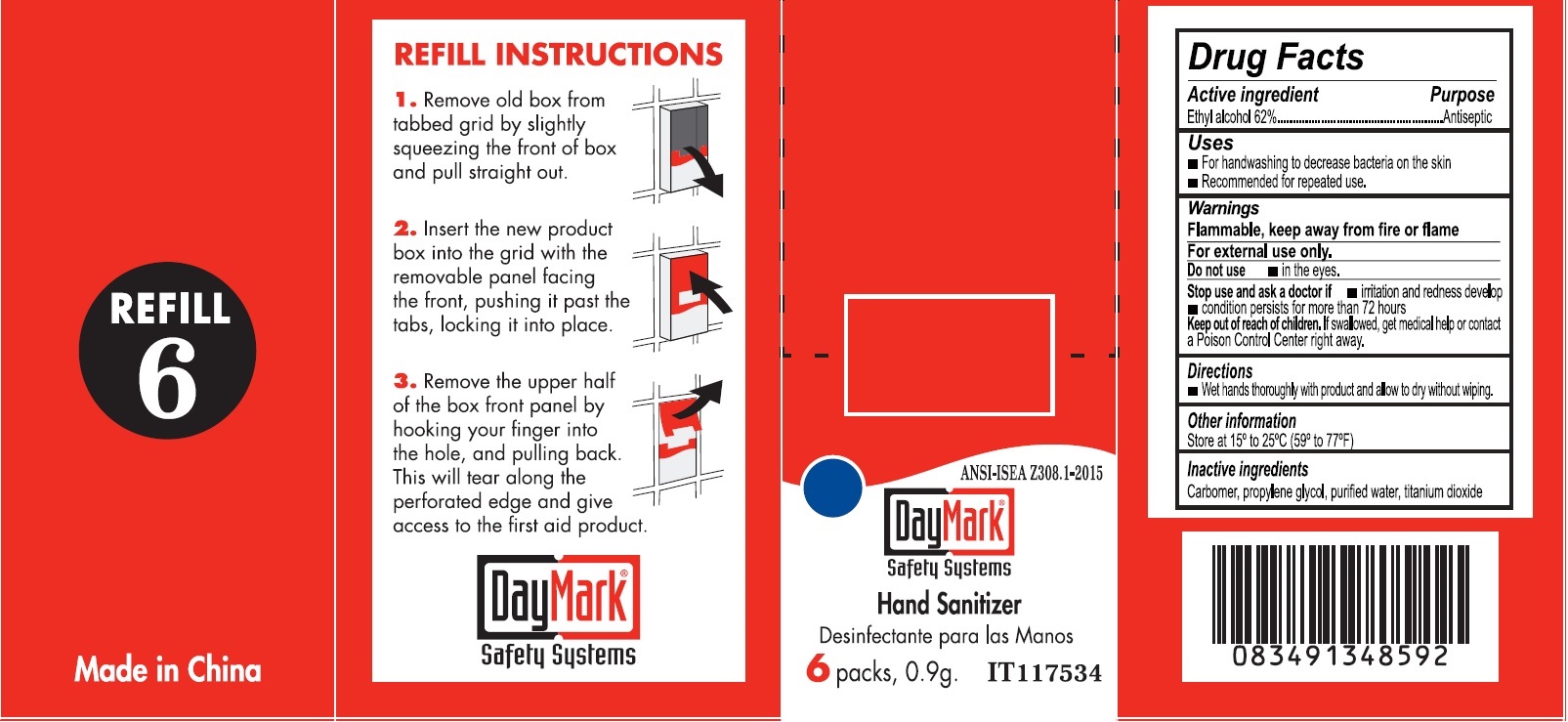
- Drug Facts - Hand Sanitizer
- Active ingredient
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
- Antiseptic Towelettes (49687-0016-0) Labeling:
- Alcohol Cleansing Pads (49687-0012-0) Labeling:
- Antibiotic Application (49687-0013-0) Labeling:
- Burn Treatment (49687-0014-0) Labeling:
- Hand Sanitizer (49687-0015-0) Labeling:
- Large ANSI First Aid Kit (49687-0021-0) Labeling:
- Eye Wash (49687-0010-0) Labeling:
-
INGREDIENTS AND APPEARANCE
LARGE ANSI FIRST AID KIT
water, benzalkonium chloride, isopropyl alcohol, bacitracin zinc, neomycin sulfate, polymyxin b sulfate, lidocaine hydrochloride kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 49687-0021 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 49687-0021-0 1 in 1 KIT 08/09/2016 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 TUBE 30 mL Part 2 20 PATCH 18 g Part 3 20 POUCH 18 g Part 4 6 PACKAGE 5 g Part 5 10 POUCH 9 g Part 6 6 PACKAGE 5.4 g Part 1 of 6 EYE WASH
water solutionProduct Information Item Code (Source) NDC: 49687-0010 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 991 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 49687-0010-1 1 in 1 BOX 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part349 08/09/2016 Part 2 of 6 ANTISEPTIC TOWELETTES
benzalkonium chloride clothProduct Information Item Code (Source) NDC: 49687-0011 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 2 in 1 BOX 1 NDC: 49687-0011-1 10 in 1 BOX 1 0.9 g in 1 PATCH; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 08/09/2016 Part 3 of 6 ALCOHOL CLEANSING
isopropyl alcohol clothProduct Information Item Code (Source) NDC: 49687-0012 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 70 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 49687-0012-0 20 in 1 KIT 1 0.9 g in 1 POUCH; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A Part 4 of 6 ANTIBIOTIC APPLICATION
bacitracin zinc, neomycin sulfate, polymyxin b sulfate ointmentProduct Information Item Code (Source) NDC: 49687-0013 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BACITRACIN ZINC (UNII: 89Y4M234ES) (BACITRACIN - UNII:58H6RWO52I) BACITRACIN 400 [iU] in 1 g NEOMYCIN SULFATE (UNII: 057Y626693) (NEOMYCIN - UNII:I16QD7X297) NEOMYCIN 3.5 mg in 1 g POLYMYXIN B SULFATE (UNII: 19371312D4) (POLYMYXIN B - UNII:J2VZ07J96K) POLYMYXIN B 5000 [iU] in 1 g Inactive Ingredients Ingredient Name Strength MINERAL OIL (UNII: T5L8T28FGP) PETROLATUM (UNII: 4T6H12BN9U) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 49687-0013-0 10 in 1 KIT 1 0.9 g in 1 PACKAGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333B Part 5 of 6 BURN TREATMENT
benzalkonium chloride, lidocaine hydrochloride creamProduct Information Item Code (Source) NDC: 49687-0014 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 g LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE 0.5 g in 100 g Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 49687-0014-0 10 in 1 KIT 1 0.9 g in 1 POUCH; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 08/09/2016 Part 6 of 6 HAND SANITIZER
alcohol gelProduct Information Item Code (Source) NDC: 49687-0015 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 0.62 g in 1 g Inactive Ingredients Ingredient Name Strength CARBOXYPOLYMETHYLENE (UNII: 0A5MM307FC) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 49687-0015-1 6 in 1 BOX 1 0.9 g in 1 PACKAGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 08/09/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333 08/09/2016 Labeler - CMC Group, Inc. (005583328)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.