VYFEMLA- norethindrone and ethinyl estradiol kit
Vyfemla by
Drug Labeling and Warnings
Vyfemla by is a Prescription medication manufactured, distributed, or labeled by LUPIN LIMITED. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Vyfemla™ (norethindrone and ethinyl estradiol tablets USP) provides a continuous regimen for oral contraception derived from 21 light peach tablets composed of norethindrone and ethinyl estradiol to be followed by 7 white tablets of inert ingredients. The structural formulas are:
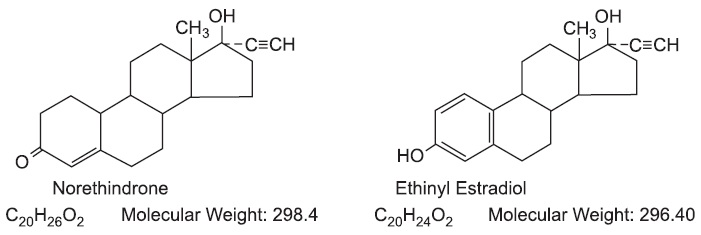
The light peach active Vyfemla (norethindrone and ethinyl estradiol tablets USP) contains 0.4 mg norethindrone and 0.035 mg ethinyl estradiol and contain the following inactive ingredients: FD&C yellow No. 6 (aluminum lake), lactose anhydrous, lactose monohydrate, magnesium stearate, povidone, and sodium starch glycolate. The white tablets in the 28 Day regimen contains only inert ingredients as follows croscarmellose sodium, lactose monohydrate, magnesium stearate and microcrystalline cellulose.
-
CLINICAL PHARMACOLOGY
Combination oral contraceptives act by suppression of gonadotropins. Although the primary mechanism of this action is inhibition of ovulation, other alterations include changes in the cervical mucus (which increase the difficulty of sperm entry into the uterus) and the endometrium (which reduce the likelihood of implantation).
-
INDICATIONS AND USAGE
Oral contraceptives are indicated for the prevention of pregnancy in women who elect to use this product as a method of contraception.
Oral contraceptives are highly effective. Table 1 lists the typical accidental pregnancy rates for users of combination oral contraceptives and other methods of contraception. The efficacy of these contraceptive methods, except sterilization, depends upon the reliability with which they are used. Correct and consistent use of methods can result in lower failure rates.
TABLE 1 LOWEST EXPECTED AND TYPICAL FAILURE RATES DURING THE FIRST YEAR OF CONTINUOUS USE OF A METHOD % of Women Experiencing an Accidental Pregnancy in the First Year of Continuous Use Reproduced with permission of the Population Council from J. Trussell, et al: Contraceptive failure in the United States: An update.
Studies in Family Planning, 21(1), January-February 1990.
- * The authors’ best guess of the percentage of women expected to experience an accidental pregnancy among couples who initiate a method (not necessarily for the first time) and who use it consistently and correctly during the first year if they do not stop for any reason other than pregnancy.
- † This term represents “typical” couples who initiate use of a method (not necessarily for the first time), who experience an accidental pregnancy during the first year if they do not stop use for any reason other than pregnancy.
- ‡ Combined typical rate for both combined and progestin only.
- § Combined typical rate for both medicated and nonmedicated IUD.
Method
Lowest Expected*
Typical†
(No contraception)
(85)
(85)
Oral contraceptives
combined
0.1
3‡
progestin only
0.5
3‡
Diaphragm with spermicidal cream or jelly
6
18
Spermicides alone
(foam, creams, jellies and vaginal suppositories)
3
21
Vaginal sponge
nulliparous
6
18
multiparous
9
28
IUD
0.8 to 2.0
3§
Condom without spermicides
2
12
Periodic abstinence (all methods)
1 to 9
20
Injectable progestogen
0.3 to 0.4
0.3 to 0.4
Implants
6 capsules
0.04
0.04
2 rods
0.03
0.03
Female sterilization
0.2
0.4
Male sterilization
0.1
0.15
-
CONTRAINDICATIONS
Oral contraceptives should not be used in women who currently have the following conditions:
- Thrombophlebitis or thromboembolic disorders
- A past history of deep vein thrombophlebitis or thromboembolic disorders
- Cerebrovascular or coronary artery disease
- Known or suspected carcinoma of the breast
- Carcinoma of the endometrium or other known or suspected estrogen-dependent neoplasia
- Undiagnosed abnormal genital bleeding
- Cholestatic jaundice of pregnancy or jaundice with prior pill use
- Hepatic adenomas or carcinomas
- Known or suspected pregnancy
-
WARNINGS
Cigarette smoking increases the risk of serious cardiovascular side effects from oral contraceptive use. This risk increases with age and with heavy smoking (15 or more cigarettes per day) and is quite marked in women over 35 years of age. Women who use oral contraceptives should be strongly advised not to smoke.
The use of oral contraceptives is associated with increased risk of several serious conditions including myocardial infarction, thromboembolism, stroke, hepatic neoplasia, and gallbladder disease, although the risk of serious morbidity or mortality is very small in healthy women without underlying risk factors. The risk of morbidity and mortality increases significantly in the presence of other underlying risk factors such as hypertension, hyperlipidemias, obesity and diabetes.
Practitioners prescribing oral contraceptives should be familiar with the following information relating to these risks.
The information contained in this package insert is principally based on studies carried out in patients who used oral contraceptives with higher formulations of estrogens and progestogens than those in common use today. The effect of long-term use of the oral contraceptives with lower formulations of both estrogens and progestogens remains to be determined.
Throughout this labeling, epidemiological studies reported are of two types: retrospective or case control studies and prospective or cohort studies. Case control studies provide a measure of the relative risk of a disease, namely, a ratio of the incidence of a disease among oral contraceptive users to that among nonusers. The relative risk does not provide information on the actual clinical occurrence of a disease. Cohort studies provide a measure of attributable risk, which is the difference in the incidence of disease between oral contraceptive users and nonusers. The attributable risk does provide information about the actual occurrence of a disease in the population*. For further information, the reader is referred to a text on epidemiological methods.
*Adapted from Stadel BB: Oral contraceptives and cardiovascular disease. N Engl J Med, 1981;305:612-618, 672-677; with author's permission.
1. Thromboembolic Disorders and Other Vascular Problems
The physician should be alert to the earliest manifestations of thromboembolic thrombotic disorders as discussed below. Should any of these occur or be suspected the drug should be discontinued immediately.
a. Myocardial Infarction
An increased risk of myocardial infarction has been attributed to oral contraceptive use. This risk is primarily in smokers or women with other underlying risk factors for coronary artery disease such as hypertension, hypercholesterolemia, morbid obesity, and diabetes. The relative risk of heart attack for current oral contraceptive users has been estimated to be two to six. The risk is very low under the age of 30.
Smoking in combination with oral contraceptive use has been shown to contribute substantially to the incidence of myocardial infarctions in women in their mid-thirties or older, with smoking accounting for the majority of excess cases. Mortality rates associated with circulatory disease have been shown to increase substantially in smokers over the age of 35 and nonsmokers over the age of 40 (Figure 1) among women who use oral contraceptives.
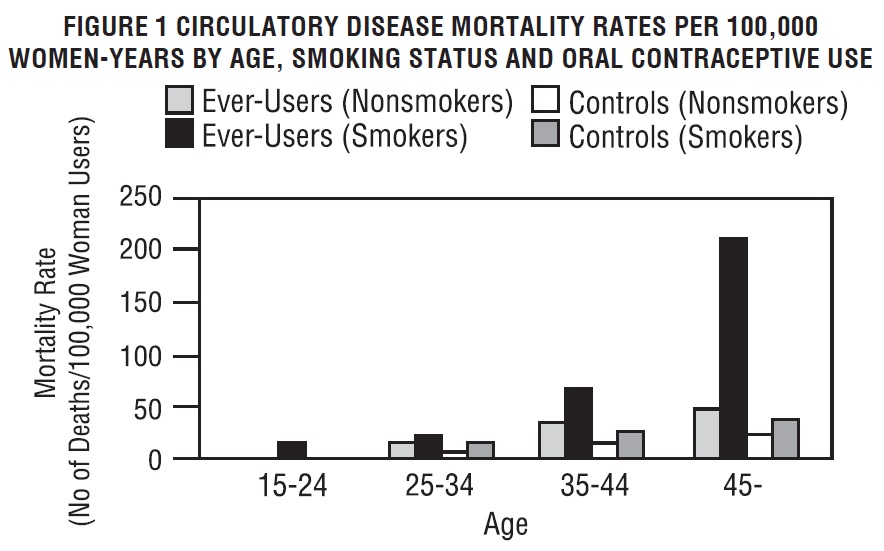
Layde PM, Beral V: Further analyses of mortality in oral contraceptive users: Royal College of General Practitioners' oral contraception study. (Table 5) Lancet 1981; 1:541-546.
Oral contraceptives may compound the effects of well-known risk factors, such as hypertension, diabetes, hyperlipidemias, age and obesity. In particular, some progestogens are known to decrease HDL cholesterol and cause glucose intolerance, while estrogens may create a state of hyperinsulinism. Oral contraceptives have been shown to increase blood pressure among users [see section 9 in WARNINGS]. Such increases in risk factors have been associated with an increased risk of heart disease and the risk increases with the number of risk factors present. Oral contraceptives must be used with caution in women with cardiovascular disease risk factors.
b. Thromboembolism
An increased risk of thromboembolic and thrombotic disease associated with the use of oral contraceptives is well established. Case control studies have found the relative risk of users compared to non-users to be 3 for the first episode of superficial venous thrombosis, 4 to 11 for deep vein thrombosis or pulmonary embolism, and 1.5 to 6 for women with predisposing conditions for venous thromboembolic disease. Cohort studies have shown the relative risk to be somewhat lower, about 3 for new cases and about 4.5 for new cases requiring hospitalization. The risk of thromboembolic disease due to oral contraceptives is not related to length of use and disappears after pill use is stopped.
A two- to four-fold increase in relative risk of postoperative thromboembolic complications has been reported with the use of oral contraceptives. The relative risk of venous thrombosis in women who have predisposing conditions is twice that of women without such medical conditions. If feasible, oral contraceptives should be discontinued at least four weeks prior to and for two weeks after elective surgery of a type associated with an increase in risk of thromboembolism and during and following prolonged immobilization.
Since the immediate postpartum period is also associated with an increased risk of thromboembolism, oral contraceptives should be started no earlier than four to six weeks after delivery in women who elect not to breastfeed.
Oral contraceptives have been shown to increase both the relative and attributable risk of cerebrovascular events (thrombotic and hemorrhagic strokes); although, in general, the risk is greatest among older (greater than 35 years), hypertensive women who also smoke. Hypertension was found to be a risk factor for both users and non-users, for both types of strokes, while smoking interacted to increase the risk for hemorrhagic strokes.
In a large study, the relative risk of thrombotic strokes has been shown to range from 3 for normotensive users to 14 for users with severe hypertension. The relative risk of hemorrhagic stroke is reported to be 1.2 for nonsmokers who used oral contraceptives, 2.6 for smokers who did not use oral contraceptives, 7.6 for smokers who used oral contraceptives, 1.8 for normotensive users and 25.7 for users with severe hypertension. The attributable risk is also greater in older women.
d. Dose-Related Risk of Vascular Disease from Oral Contraceptives
A positive association has been observed between the amount of estrogen and progestogen in oral contraceptives and the risk of vascular disease. A decline in serum high density lipoproteins (HDL) has been reported with many progestational agents. A decline in serum high density lipoproteins has been associated with an increased incidence of ischemic heart disease. Because estrogens increase HDL cholesterol, the net effect of an oral contraceptive depends on a balance achieved between doses of estrogen and progestogen and the nature and absolute amount of progestogens used in the contraceptive. The amount of both hormones should be considered in the choice of an oral contraceptive.
Minimizing exposure to estrogen and progestogen is in keeping with good principles of therapeutics. For any particular estrogen/ progestogen combination, the dosage regimen prescribed should be one which contains the least amount of estrogen and progestogen that is compatible with a low failure rate and the needs of the individual patient. New acceptors of oral contraceptive agents should be started on preparations containing 0.05 mg or less of estrogen.
e. Persistence of Risk
There are two studies which have shown persistence of risk of vascular disease for ever-users of oral contraceptives. In a study in the United States, the risk of developing myocardial infarction after discontinuing oral contraceptives persists for at least 9 years for women 40 to 49 years old who had used oral contraceptives for five or more years, but this increased risk was not demonstrated in other age groups. In another study in Great Britain, the risk of developing cerebrovascular disease persisted for at least six years after discontinuation of oral contraceptives, although excess risk was very small. However, both studies were performed with oral contraceptive formulations containing 50 micrograms or higher of estrogens.
2. Estimates of Mortality from Contraceptive Use
One study gathered data from a variety of sources which have estimated the mortality rate associated with different methods of contraception at different ages (Table 2).
TABLE 2 ANNUAL NUMBER OF BIRTH-RELATED OR METHOD-RELATED DEATHS ASSOCIATED WITH CONTROL OF FERTILITY PER 100,000 NONSTERILE WOMEN, BY FERTILITY CONTROL METHOD ACCORDING TO AGE Ory HW: Mortality associated with fertility and fertility control: 1983. Fam Plann Perspect 1983; 15:50-56.
- * Deaths are birth related.
- † Deaths are method related.
Method of Control and Outcome
AGE
15 to 19
20 to 24
25 to 29
30 to 34
35 to 39
40 to 44
No fertility control methods*
7.0
7.4
9.1
14.8
25.7
28.2
Oral contraceptives nonsmoker†
0.3
0.5
0.9
1.9
13.8
31.6
Oral contraceptives smoker†
2.2
3.4
6.6
13.5
51.1
117.2
IUD†
0.8
0.8
1.0
1.0
1.4
1.4
Condom*
1.1
1.6
0.7
0.2
0.3
0.4
Diaphragm/spermicide*
1.9
1.2
1.2
1.3
2.2
2.8
Periodic abstinence*
2.5
1.6
1.6
1.7
2.9
3.6
These estimates include the combined risk of death associated with contraceptive methods plus the risk attributable to pregnancy in the event of method failure. Each method of contraception has its specific benefits and risk. The study concluded that with the exception of oral contraceptive users 35 and older who smoke and 40 and older who do not smoke, mortality associated with all methods of birth control is low and below that associated with childbirth.
The observation of a possible increase in risk of mortality with age for oral contraceptive users is based on data gathered in the 1970's– but not reported until 1983. However, current clinical practice involves the use of lower estrogen dose formulations combined with careful restriction of oral contraceptive use to women who do not have the various risk factors listed in this labeling.
Because of these changes in practice and, also, because of some limited new data which suggest that the risk of cardiovascular disease with the use of oral contraceptives may now be less than previously observed (Porter JB, Hunter J, Jick H, et al. Oral contraceptives and nonfatal vascular disease. Obstet Gynecol 1985;66:1-4 and Porter JB, Jick H, Walker AM. Mortality among oral contraceptive users. Obstet Gynecol 1987;70:29-32), the Fertility and Maternal Health Drugs Advisory Committee was asked to review the topic in 1989. The Committee concluded that although cardiovascular disease risk may be increased with oral contraceptive use after age 40 in healthy nonsmoking women (even with the newer low-dose formulations), there are greater potential health risks associated with pregnancy in older women and with the alternative surgical and medical procedures which may be necessary if such women do not have access to effective and acceptable means of contraception.
Therefore, the Committee recommended that the benefits of oral contraceptive use by healthy nonsmoking women over 40 may outweigh the possible risks. Of course, older women, as all women who take oral contraceptives, should take the lowest possible dose formulation that is effective.
3. Carcinoma of the Reproductive Organs
Numerous epidemiological studies have been performed on the incidence of breast, endometrial, ovarian, and cervical cancer in women using oral contraceptives. The overwhelming evidence in the literature suggests that use of oral contraceptives is not associated with an increase in the risk of developing breast cancer, regardless of the age and parity of first use or with most of the marketed brands and doses. The Cancer and Steroid Hormone (CASH) study also showed no latent effect on the risk of breast cancer for at least a decade following long-term use. A few studies have shown a slightly increased relative risk of developing breast cancer, although the methodology of these studies, which included differences in examination of users and nonusers and differences in age at start of use, has been questioned.
Some studies suggest that oral contraceptive use has been associated with an increase in the risk of cervical intraepithelial neoplasia in some populations of women.
However, there continues to be controversy about the extent to which such findings may be due to differences in sexual behavior and other factors.
In spite of many studies of the relationship between oral contraceptive use and breast cancer and cervical cancers, a cause-and-effect relationship has not been established.
4. Hepatic Neoplasia
Benign hepatic adenomas are associated with oral contraceptive use, although their occurrence is rare in the United States. Indirect calculations have estimated the attributable risk to be in the range of 3.3 cases/100,000 for users, a risk that increases after four or more years of use. Rupture of hepatic adenomas may cause death through intra-abdominal hemorrhage.
Studies from Britain have shown an increased risk of developing hepatocellular carcinoma in long-term (greater than 8 years) oral contraceptive users. However, these cancers are extremely rare in the U.S. and the attributable risk (the excess incidence) of liver cancers in oral contraceptive users approaches less than one per million users.
5. Ocular Lesions
There have been clinical case reports of retinal thrombosis associated with the use of oral contraceptives. Oral contraceptives should be discontinued if there is unexplained partial or complete loss of vision; onset of proptosis or diplopia; papilledema; or retinal vascular lesions. Appropriate diagnostic and therapeutic measures should be undertaken immediately.
6. Oral Contraceptive Use Before or During Early Pregnancy
Extensive epidemiological studies have revealed no increased risk of birth defects in women who have used oral contraceptives prior to pregnancy. Studies also do not suggest a teratogenic effect, particularly in so far as cardiac anomalies and limb reduction defects are concerned, when taken inadvertently during early pregnancy.
The administration of oral contraceptives to induce withdrawal bleeding should not be used as a test for pregnancy. Oral contraceptives should not be used during pregnancy to treat threatened or habitual abortion.
It is recommended that for any patient who has missed two consecutive periods, pregnancy should be ruled out before continuing oral contraceptive use. If the patient has not adhered to the prescribed schedule, the possibility of pregnancy should be considered at the time of the first missed period. Oral contraceptive use should be discontinued if pregnancy is confirmed.
7. Gallbladder Disease
Earlier studies have reported an increased lifetime relative risk of gallbladder surgery in users of oral contraceptives and estrogens. More recent studies, however, have shown that the relative risk of developing gallbladder disease among oral contraceptive users may be minimal.
The recent findings of minimal risk may be related to the use of oral contraceptive formulations containing lower hormonal doses of estrogens and progestogens.
8. Carbohydrate and Lipid Metabolic Effects
Oral contraceptives have been shown to cause glucose intolerance in a significant percentage of users. Oral contraceptives containing greater than 75 micrograms of estrogens cause hyperinsulinism, while lower doses of estrogen cause less glucose intolerance. Progestogens increase insulin secretion and create insulin resistance, this effect varying with different progestational agents.
However, in the nondiabetic woman, oral contraceptives appear to have no effect on fasting blood glucose. Because of these demonstrated effects, prediabetic and diabetic women should be carefully observed while taking oral contraceptives.
A small proportion of women will have persistent hypertriglyceridemia while on the pill. As discussed earlier [see WARNINGS 1.a. 1.d.], changes in serum triglycerides and lipoprotein levels have been reported in oral contraceptive users.
9. Elevated Blood Pressure
An increase in blood pressure has been reported in women taking oral contraceptives and this increase is more likely in older oral contraceptive users and with continued use. Data from the Royal College of General Practitioners and subsequent randomized trials have shown that the incidence of hypertension increases with increasing concentrations of progestogens.
Women with a history of hypertension or hypertension-related diseases, or renal disease should be encouraged to use another method of contraception. If women elect to use oral contraceptives, they should be monitored closely and if significant elevation of blood pressure occurs, oral contraceptives should be discontinued. For most women, elevated blood pressure will return to normal after stopping oral contraceptives, and there is no difference in the occurrence of hypertension among ever- and never-users.
10. Headache
The onset or exacerbation of migraine or development of headache with a new pattern which is recurrent, persistent or severe requires discontinuation of oral contraceptives and evaluation of the cause.
11. Bleeding Irregularities
Breakthrough bleeding and spotting are sometimes encountered in patients on oral contraceptives, especially during the first three months of use. Nonhormonal causes should be considered and adequate diagnostic measures taken to rule out malignancy or pregnancy in the event of breakthrough bleeding, as in the case of any abnormal vaginal bleeding. If pathology has been excluded, time or a change to another formulation may solve the problem. In the event of amenorrhea, pregnancy should be ruled out.
Women with a history of oligomenorrhea or secondary amenorrhea or young women without regular cycles prior to taking oral contraceptives may again have irregular bleeding or amenorrhea after discontinuation of oral contraceptives.
-
PRECAUTIONS
1. Sexually-Transmitted Diseases
Patients should be counseled that this product does not protect against HIV infection (AIDS) and other sexually transmitted diseases.
2. Physical Examination and Follow-Up
It is good medical practice for all women to have annual history and physical examinations, including women using oral contraceptives. The physical examination, however, may be deferred until after initiation of oral contraceptives if requested by the woman and judged appropriate by the clinician. The physical examination should include special reference to blood pressure, breasts, abdomen and pelvic organs, including cervical cytology, and relevant laboratory tests. In case of undiagnosed, persistent or recurrent abnormal vaginal bleeding, appropriate measures should be conducted to rule out malignancy. Women with a strong family history of breast cancer or who have breast nodules should be monitored with particular care.
3. Lipid Disorders
Women who are being treated for hyperlipidemias should be followed closely if they elect to use oral contraceptives. Some progestogens may elevate LDL levels and may render the control of hyperlipidemias more difficult.
4. Liver Function
If jaundice develops in any woman receiving such drugs, the medication should be discontinued. Steroid hormones may be poorly metabolized in patients with impaired liver function.
5. Fluid Retention
Oral contraceptives may cause some degree of fluid retention. They should be prescribed with caution, and only with careful monitoring, in patients with conditions which might be aggravated by fluid retention.
6. Emotional Disorders
Women with a history of depression should be carefully observed and the drug discontinued if depression recurs to a serious degree.
Patients becoming significantly depressed while taking oral contraceptives should stop the medication and use an alternate method of contraception in an attempt to determine whether the symptom is drug related.
7. Contact Lenses
Contact lens wearers who develop visual changes or changes in lens tolerance should be assessed by an ophthalmologist.
8. Drug Interactions
Reduced efficacy and increased incidence of breakthrough bleeding and menstrual irregularities have been associated with concomitant use of rifampin. A similar association, though less marked, has been suggested with barbiturates, phenylbutazone, phenytoin sodium, and possibly with griseofulvin, ampicillin, and tetracyclines.
9. Interactions with Laboratory Tests
Certain endocrine and liver function tests and blood components may be affected by oral contraceptives:
- Increased prothrombin and factors VII, VIII, IX, and X; decreased antithrombin 3; increased norepinephrine-induced platelet aggregability.
- Increased thyroid-binding globulin (TBG) leading to increased circulating total thyroid hormone, as measured by protein-bound iodine (PBI), T4 by column or by radioimmunoassay. Free T3 resin uptake is decreased, reflecting the elevated TBG, free T4 concentration is unaltered.
- Other binding proteins may be elevated in serum.
- Sex-binding globulins are increased and result in elevated levels of total circulating sex steroids and corticoids; however, free or biologically active levels remain unchanged.
- Triglycerides may be increased.
- Glucose tolerance may be decreased.
- Serum folate levels may be depressed by oral contraceptive therapy. This may be of clinical significance if a woman becomes pregnant shortly after discontinuing oral contraceptives.
See WARNINGS section.
11. Pregnancy
Pregnancy Category X
See CONTRAINDICATIONS and WARNINGS sections.
12. Nursing Mothers
Small amounts of oral contraceptive steroids have been identified in the milk of nursing mothers and a few adverse effects on the child have been reported, including jaundice and breast enlargement. In addition, oral contraceptives given in the postpartum period may interfere with lactation by decreasing the quantity and quality of breast milk. If possible, the nursing mother should be advised not to use oral contraceptives but to use other forms of contraception until she has completely weaned her child.
13. Vomiting and/or Diarrhea
Although a cause-and-effect relationship has not been clearly established, several cases of oral contraceptive failure have been reported in association with vomiting and/or diarrhea. If significant gastrointestinal disturbance occurs in any woman receiving contraceptive steroids, the use of a back-up method of contraception for the remainder of that cycle is recommended.
14. Pediatric Use
Safety and efficacy of Vyfemla (norethindrone and ethinyl estradiol tablets USP) have been established in women of reproductive age. Safety and efficacy are expected to be the same in postpubertal adolescents under the age of 16 years and in users ages 16 years and older. Use of this product before menarche is not indicated.
- SPL UNCLASSIFIED SECTION
-
ADVERSE REACTIONS
An increased risk of the following serious adverse reactions has been associated with the use of oral contraceptives [see WARNINGS section]:
- Thrombophlebitis
- Arterial thromboembolism
- Pulmonary embolism
- Myocardial infarction
- Cerebral hemorrhage
- Cerebral thrombosis
- Hypertension
- Gallbladder disease
- Hepatic adenomas or benign liver tumors
There is evidence of an association between the following conditions and the use of oral contraceptives, although additional confirmatory studies are needed:
- Mesenteric thrombosis
- Retinal thrombosis
The following adverse reactions have been reported in patients receiving oral contraceptives and are believed to be drug-related:
- Nausea
- Vomiting
- Gastrointestinal symptoms (such as abdominal cramps and bloating)
- Breakthrough bleeding
- Spotting
- Change in menstrual flow
- Amenorrhea
- Temporary infertility after discontinuation of treatment
- Edema
- Melasma which may persist
- Breast changes: tenderness, enlargement, and secretion
- Change in weight (increase or decrease)
- Change in cervical ectropion and secretion
- Possible diminution in lactation when given immediately postpartum
- Cholestatic jaundice
- Migraine
- Rash (allergic)
- Mental depression
- Reduced tolerance to carbohydrates
- Vaginal candidiasis
- Change in corneal curvature (steepening)
- Intolerance to contact lenses
The following adverse reactions have been reported in users of oral contraceptives, and the association has been neither confirmed nor refuted:
- Premenstrual syndrome
- Cataracts
- Changes in appetite
- Cystitis-like syndrome
- Headache
- Nervousness
- Dizziness
- Hirsutism
- Loss of scalp hair
- Erythema multiforme
- Erythema nodosum
- Hemorrhagic eruption
- Vaginitis
- Porphyria
- Impaired renal function
- Hemolytic uremic syndrome
- Budd-Chiari syndrome
- Acne
- Changes in libido
- Colitis
-
OVERDOSAGE
Serious ill effects have not been reported following acute ingestion of large doses of oral contraceptives by young children.
Overdosage may cause nausea, and withdrawal bleeding may occur in females.
NONCONTRACEPTIVE HEALTH BENEFITS
The following noncontraceptive health benefits related to the use of oral contraceptives are supported by epidemiological studies which largely utilized oral contraceptive formulations containing estrogen doses exceeding 0.035 mg of ethinyl estradiol or 0.05 mg of mestranol.
Effects on menses:
- Increased menstrual cycle regularity
- Decreased blood loss and decreased incidence of iron deficiency anemia
- Decreased incidence of dysmenorrhea
Effects related to inhibition of ovulation:
- Decreased incidence of functional ovarian cysts
- Decreased incidence of ectopic pregnancies
- Decreased incidence of fibroadenomas and fibrocystic disease of the breast
- Decreased incidence of acute pelvic inflammatory disease
- Decreased incidence of endometrial cancer
- Decreased incidence of ovarian cancer
-
DOSAGE AND ADMINISTRATION
The following is a summary of the instructions given to the patient in the "HOW TO TAKE THE PILL" section of the DETAILED PATIENT PACKAGE LABELING.
The patient is given instructions in five (5) categories:
- IMPORTANT POINTS TO REMEMBER: The patient is told (a) that she should take one pill every day at the same time, (b) many women have spotting or light bleeding or gastric distress during the first one to three cycles, (c) missing pills can also cause spotting or light bleeding, (d) she should use a back-up method for contraception if she has vomiting or diarrhea or takes some concomitant medications, and/or if she has trouble remembering the pill, (e) if she has any other questions, she should consult her physician.
- BEFORE SHE STARTS TAKING HER PILLS : She should decide what time of day she wishes to take the pill, check whether her pill pack has 28 pills, and note the order in which she should take the pills (diagrammatic drawings of the pill pack are included in the patient insert).
- WHEN SHE SHOULD START THE FIRST PACK: The Day-One start is listed as the first choice and the Sunday start (the Sunday after her period starts) is given as the second choice. If she uses the Sunday start she should use a back-up method in the first cycle if she has intercourse before she has taken seven pills.
- WHAT TO DO DURING THE CYCLE: The patient is advised to take one pill at the same time every day until the pack is empty. If she is on the 28 day regimen, she should start the next pack the day after the last inactive tablet and not wait any days between packs.
- WHAT TO DO IF SHE MISSES A PILL OR PILLS: The patient is given instructions about what she should do if she misses one, two or more than two pills at varying times in her cycle for both the Day-One and the Sunday start. The patient is warned that she may become pregnant if she has unprotected intercourse in the seven days after missing pills. To avoid this, she must use another birth control method such as condom, foam, or sponge in these seven days.
-
HOW SUPPLIED
Vyfemla (norethindrone and ethinyl estradiol tablets USP) is available in wallet (NDC: 68180-875-11) containing 28 tablets packed in a pouch (NDC: 68180-875-11). Such three pouches are packaged in a carton (NDC: 68180-875-13).
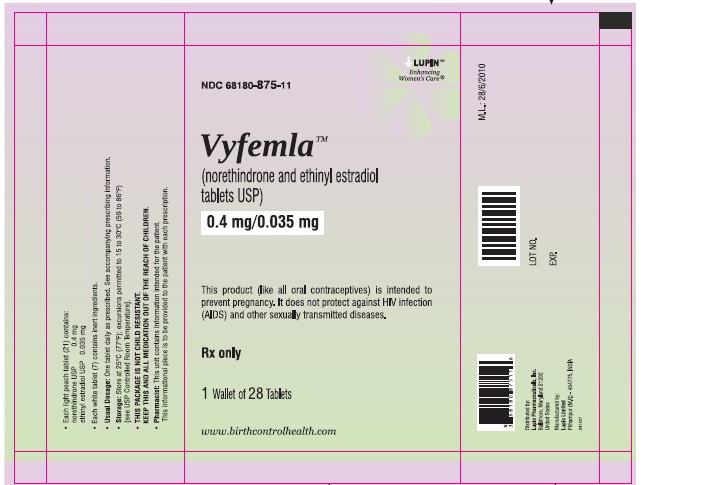
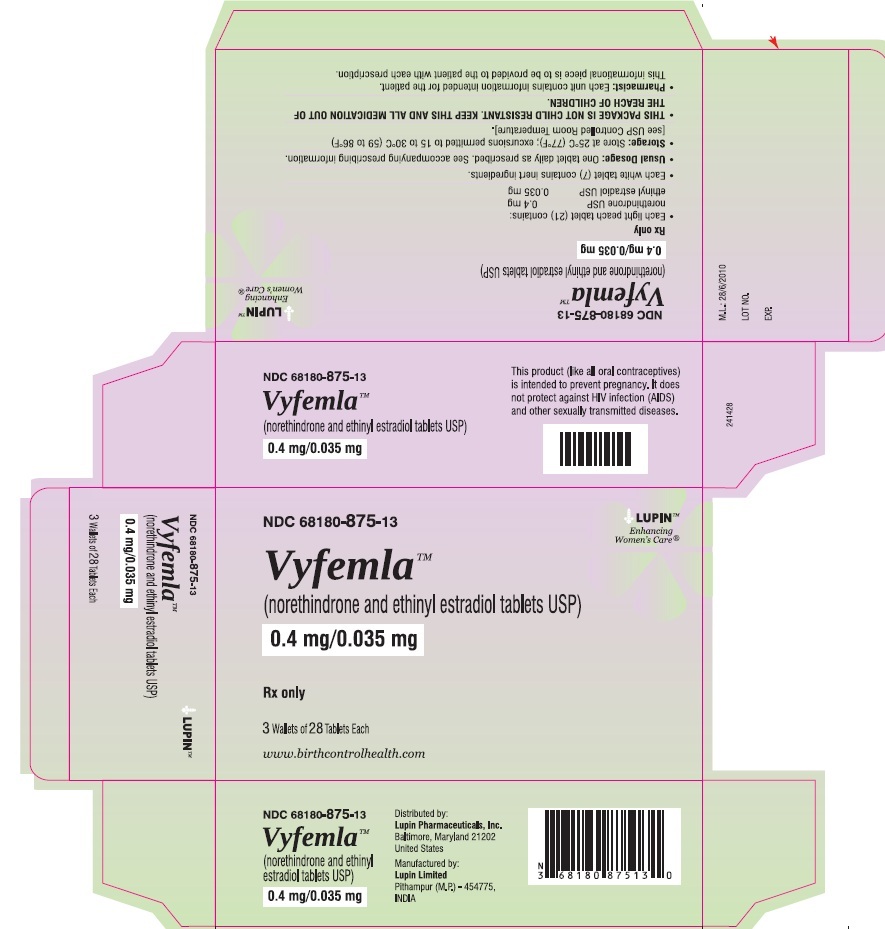
- Each of the 21 light peach colored, round, flat face beveled edged tablets debossed with "LU" on one side and "I25" on the other side containing 0.4 mg norethindrone and 0.035 mg ethinyl estradiol.
- Each of the 7 white to off-white, round, biconvex tablets debossed with "LU" on one side and "U22" on other side containing inert ingredients.
Store at 25°C (77°F); excursions permitted to 15 to 30°C (59 to 86°F). [See USP Controlled Room Temperature].
References are available upon request.
Distributed by:
Lupin Pharmaceuticals, Inc.
Baltimore, Maryland 21202
United States
Manufactured by:
Lupin Limited
Pithampur (M.P.) - 454 775
INDIA
September 2015 ID#240525
-
SPL UNCLASSIFIED SECTION
(norethindrone and ethinyl estradiol tablets USP, 0.4 mg/0.035 mg)
Rx Only
BRIEF SUMMARY PATIENT PACKAGE INSERT
This product (like all oral contraceptives) is intended to prevent pregnancy. It does not protect against HIV infection (AIDS) and other sexually transmitted diseases.
Oral contraceptives, also known as "birth control pills" or "the pill," are taken to prevent pregnancy and when taken correctly, have a failure rate of about 1% per year when used without missing any pills. The typical failure rate of large numbers of pill users is less than 3% per year when women who miss pills are included.
Oral contraceptive use is associated with certain serious diseases that can be life-threatening or may cause temporary or permanent disability. The risks associated with taking oral contraceptives increase significantly if you:
- Smoke
- Have high blood pressure, diabetes, high cholesterol
- Have or have had clotting disorders, heart attack, stroke, angina pectoris, cancer of the breast or sex organs, jaundice or malignant or benign liver tumors.
You should not take the pill if you suspect you are pregnant or have unexplained vaginal bleeding.
Cigarette smoking increases the risk of serious cardiovascular side effects from oral contraceptive use. This risk increases with age and with heavy smoking (15 or more cigarettes per day) and is quite marked in women over 35 years of age. Women who use oral contraceptives should be strongly advised not to smoke.
Most side effects of the pill are not serious. The most common side effects are nausea, vomiting, bleeding between menstrual periods, weight gain, breast tenderness, and difficulty wearing contact lenses. These side effects, especially nausea and vomiting, may subside within the first three months of use.
The serious side effects of the pill occur very infrequently, especially if you are in good health and are young. However, you should know that the following medical conditions have been associated with or made worse by the pill:
- Blood clots in the legs (thrombophlebitis), lungs (pulmonary embolism), stoppage or rupture of a blood vessel in the brain (stroke), blockage of blood vessels in the heart (heart attack or angina pectoris), or other organs of the body. As mentioned above, smoking increases the risk of heart attacks and strokes and subsequent serious medical consequences.
- Liver tumors, which may rupture and cause severe bleeding. A possible but not definite association has been found with the pill and liver cancer. However, liver cancers are extremely rare. The chance of developing liver cancer from using the pill is thus even rarer.
- High blood pressure, although blood pressure usually returns to normal when the pill is stopped.
The symptoms associated with these serious side effects are discussed in the detailed leaflet given to you with your supply of pills. Notify your doctor or health care provider if you notice any unusual physical disturbances while taking the pill. In addition, drugs such as rifampin, as well as some anticonvulsants and some antibiotics may decrease oral contraceptive effectiveness.
Studies to date of women taking the pill have not shown an increase in the incidence of cancer of the breast or cervix. There is, however, insufficient evidence to rule out the possibility that the pill may cause such cancers.
Taking the pill provides some important noncontraceptive effects. These include less painful menstruation, less menstrual blood loss and anemia, fewer pelvic infections, and fewer cancers of the ovary and the lining of the uterus.
Be sure to discuss any medical condition you may have with your health care provider. Your health care provider will take a medical and family history before prescribing oral contraceptives and will examine you. The physical examination may be delayed to another time if you request it and the health care provider believes that it is a good medical practice to postpone it. You should be reexamined at least once a year while taking oral contraceptives. The detailed patient labeling booklet gives you further information which you should read and discuss with your health care professional.
DOSAGE AND ADMINISTRATION
The instructions given in the COMBINATION DETAILED PATIENT LABELING AND BRIEF SUMMARY insert are included inside each foil pouch. The instructions include the directions on starting the first pack on Day-One (first choice) of her period and the Sunday start (Sunday after period starts). The patient is advised that, if she used the Sunday start, she should use a back-up method in the first cycle if she has intercourse before she has taken seven pills. The patient is also instructed as to what she should do if she misses a pill or pills. The patient is warned that she may become pregnant if she misses a pill or pills and that she should use a back-up method of birth control in the event she has intercourse any time during the seven day period following the missed pill or pills.
Instructions on how to use the wallet for the (28 Tablets) are included in the BRIEF SUMMARY PATIENT PACKAGE INSERT.
DETAILED PATIENT LABELING
This product (like all oral contraceptives) is intended to prevent pregnancy. It does not protect against HIV infection (AIDS) and other sexually transmitted diseases.
INTRODUCTION
Any woman who considers using oral contraceptives (the "birth control pill" or "the pill") should understand the benefits and risks of using this form of birth control.
Although the oral contraceptives have important advantages over other methods of contraception, they have certain risks that no other method has and some of these risks may continue after you have stopped using the oral contraceptive. This brochure will give you much of the information you will need to make this decision and will also help you determine if you are at risk of developing any of the serious side effects of the pill. It will tell you how to use the pill properly so that it will be as effective as possible. However, this brochure is not a replacement for a careful discussion between you and your health care professional.
You should discuss the information provided in this brochure with him or her, both when you first start taking the pill and during your revisits. You should also follow your health care professional's advice with regard to regular check-ups while you are on the pill.
EFFECTIVENESS OF ORAL CONTRACEPTIVES
Oral contraceptives or "birth control pills" or "the pill" are used to prevent pregnancy and are more effective than other nonsurgical methods of birth control. The chance of becoming pregnant is less than 1% (1 pregnancy per 100 women per year of use) when the pills are used correctly and no pills are missed. Typical failure rates are actually 3% per year. The chance of becoming pregnant increases with each missed pill during a menstrual cycle.
In comparison, typical accidental pregnancy rates for other nonsurgical methods of birth control during the first year of use are as follows:
IUD: 3%
Diaphragm with spermicides: 18%
Spermicides alone: 21%
Vaginal Sponge: 18% to 28%
Condom alone: 12%
Periodic abstinence: 20%
Injectable progestogen: 0.3% to 0.4%
Implants: 0.03% to 0.04%
No methods: 85%.
WHO SHOULD NOT TAKE ORAL CONTRACEPTIVES
Cigarette smoking increases the risk of serious cardiovascular side effects from oral contraceptive use. This risk increases with age and with heavy smoking (15 or more cigarettes per day) and is quite marked in women over 35 years of age. Women who use oral contraceptives should not smoke.
Some women should not use the pill. For example, you should not take the pill if you are pregnant or think you may be pregnant. You should also not use the pill if you have or have ever had any of the following conditions:
- A history of heart attack or stroke
- Blood clots in the legs (thrombophlebitis), lungs (pulmonary embolism), or eyes
- A history of blood clots in the deep veins of your legs
- Chest pain (angina pectoris)
- Known or suspected breast cancer or cancer of the lining of the uterus
- Unexplained vaginal bleeding (until a diagnosis is reached by your doctor)
- Yellowing of the whites of the eyes or of the skin (jaundice) during pregnancy or during previous use of the pill
- Liver tumor (benign or cancerous)
Tell your health care professional if you have ever had any of these conditions. Your health care professional can recommend a safer method of birth control.
OTHER CONSIDERATIONS BEFORE TAKING ORAL CONTRACEPTIVES
Tell your health care professional if you have:
- Breast nodules, fibrocystic disease of the breast or an abnormal breast x-ray or mammogram
- Diabetes
- Elevated cholesterol or triglycerides
- High blood pressure
- Migraine or other headaches or epilepsy
- Mental depression
- Gallbladder, heart, or kidney disease
- History of scanty or irregular menstrual periods
Women with any of these conditions should be checked often by their health care professional if they choose to use oral contraceptives.
Also, be sure to inform your doctor or health care professional if you smoke or are on any medications.
RISKS OF TAKING ORAL CONTRACEPTIVES
1. Risk of Developing Blood Clots
Blood clots and blockage of blood vessels are the most serious side effects of taking oral contraceptives. In particular, a clot in the legs can cause thrombophlebitis and a clot that travels to the lungs can cause a sudden blocking of the vessel carrying blood to the lungs. Either of these can cause death or disability. Rarely, clots occur in the blood vessels of the eye and may cause blindness, double vision, or impaired vision.
If you take oral contraceptives and need elective surgery, need to stay in bed for a prolonged illness, or have recently delivered a baby, you may be at risk of developing blood clots. You should consult your doctor about stopping oral contraceptives three to four weeks before surgery and not taking oral contraceptives for two weeks after surgery or during bed rest. You should also not take oral contraceptives soon after delivery of a baby. It is advisable to wait for at least four weeks after delivery if you are not breastfeeding. If you are breastfeeding see the section on Breastfeeding in GENERAL PRECAUTIONS.
2. Heart Attacks and Strokes
Oral contraceptives may increase the tendency to develop strokes (stoppage or rupture of blood vessels in the brain) and angina pectoris and heart attacks (blockage of blood vessels in the heart). Any of these conditions can cause death or disability. Smoking greatly increases the possibility of suffering heart attacks and strokes. Furthermore, smoking and the use of oral contraceptives greatly increase the chances of developing and dying of heart disease.
3. Gallbladder Disease
Oral contraceptive users probably have a greater risk than nonusers of having gallbladder disease, although this risk may be related to pills containing high doses of estrogens.
4. Liver Tumors
In rare cases, oral contraceptives can cause benign but dangerous liver tumors. These benign liver tumors can rupture and cause fatal internal bleeding. In addition, a possible, but not definite, association has been found with the pill and liver cancers in two studies, in which a few women who developed these very rare cancers were found to have used oral contraceptives for long periods. However, liver cancers in general are extremely rare and the chance of developing liver cancer from using the pill is thus even rarer.
5. Cancer of the Reproductive Organs
There is, at present, no confirmed evidence that oral contraceptives increase the risk of cancer of the reproductive organs and breasts in human studies. Several studies have found no overall increase in the risk of developing breast cancer. However, women who use oral contraceptives and have a strong family history of breast cancer, or who have breast nodules or abnormal mammograms, should be closely followed by their doctors.
Some studies have found an increase in the incidence of cancer of the cervix in women who use oral contraceptives. However, this finding may be related to factors other than the use of oral contraceptives.
ESTIMATED RISK OF DEATH FROM A BIRTH CONTROL METHOD OR PREGNANCY
All methods of birth control and pregnancy are associated with a risk of developing certain diseases which may lead to disability or death. An estimate of the number of deaths associated with different methods of birth control and pregnancy has been calculated and is shown in the following table.
ANNUAL NUMBER OF BIRTH-RELATED OR METHOD-RELATED DEATHS ASSOCIATED WITH CONTROL OF FERTILITY PER 100,000 NONSTERILE WOMEN, BY FERTILITY CONTROL METHOD ACCORDING TO AGE Ory HW: Mortality associated with fertility and fertility control: 1983. Fam Plann Perspect 1983; 15:50-56.
- * Deaths are birth related.
- † Deaths are method related.
Method of Control
AGE
and Outcome
15 to 19
20 to 24
25 to 29
30 to 34
35 to 39
40 to 44
No fertility control methods*
7.0
7.4
9.1
14.8
25.7
28.2
Oral contraceptives non-smoker†
0.3
0.5
0.9
1.9
13.8
31.6
Oral contraceptives smoker†
2.2
3.4
6.6
13.5
51.1
117.2
IUD†
0.8
0.8
1.0
1.0
1.4
1.4
Condom*
1.1
1.6
0.7
0.2
0.3
0.4
Diaphragm/spermicide*
1.9
1.2
1.2
1.3
2.2
2.8
Periodic abstinence*
2.5
1.6
1.6
1.7
2.9
3.6
It can be seen in the table that for women aged 15 to 39, the risk of death was highest with pregnancy (7-26 deaths per 100,000 women, depending on age). Among pill users who do not smoke, the risk of death was always lower than that associated with pregnancy for any age group, although over the age of 40, the risk increases to 32 deaths per 100,000 women, compared to 28 associated with pregnancy at that age. However, for pill users who smoke and are over the age of 35, the estimated number of deaths exceeds those for other methods of birth control. If a woman is over the age of 40 and smokes, her estimated risk of death is four times higher (117/100,000 women) than the estimated risk associated with pregnancy (28/100,000 women) in that age group.
The suggestion that women over 40 who don't smoke should not take oral contraceptives is based on information from older high-dose pills and on less selective use of pills than is practiced today. An Advisory Committee of the FDA discussed this issue in 1989 and recommended that the benefits of oral contraceptive use by healthy, nonsmoking women over 40 years of age may outweigh the possible risks. However, all women, especially older women, are cautioned to use the lowest dose pill that is effective.
In the above table, the risk of death from any birth control method is less than the risk of childbirth, except for oral contraceptive users over the age of 35 who smoke and pill users over the age of 40 even if they do not smoke.
You should discuss this information with your health care professional.
If any of these adverse conditions occur while you are taking oral contraceptives, call your doctor immediately:
- Sharp chest pain, coughing of blood, or sudden shortness of breath (indicating a possible clot in the lung)
- Pain in the calf (indicating a possible clot in the leg)
- Crushing chest pain or heaviness in the chest (indicating a possible heart attack)
- Sudden severe headache or vomiting, dizziness or fainting, disturbances of vision or speech, weakness, or numbness in an arm or leg (indicating a possible stroke)
- Sudden partial or complete loss of vision (indicating a possible clot in the eye)
- Breast lumps (indicating possible breast cancer or fibrocystic disease of the breast; ask your doctor or healthcare provider to show you how to examine your breasts)
- Severe pain or tenderness in the stomach area (indicating a possibly ruptured liver tumor)
- Difficulty in sleeping, weakness, lack of energy, fatigue, or change in mood (possibly indicating severe depression)
- Jaundice or a yellowing of the skin or eyeballs, accompanied frequently by fever, fatigue, loss of appetite, dark-colored urine, or light-colored bowel movements (indicating possible liver problems)
- Abnormal vaginal bleeding [see SIDE EFFECTS OF ORAL CONTRACEPTIVES, 1. Vaginal Bleeding below.]
SIDE EFFECTS OF ORAL CONTRACEPTIVES
In addition to the risks and more serious side effects discussed above [see RISKS OF TAKING ORAL CONTRACEPTIVES, ESTIMATED RISK OF DEATH FROM A BIRTH CONTROL METHOD OR PREGNANCY and WARNING SIGNALS sections), the following may also occur:
1. Vaginal Bleeding
Irregular vaginal bleeding or spotting may occur while you are taking the pills. Irregular bleeding may vary from slight staining between menstrual periods to breakthrough bleeding which is a flow much like a regular period. Irregular bleeding occurs most often during the first few months of oral contraceptive use, but may also occur after you have been taking the pill for some time.
Such bleeding may be temporary and usually does not indicate any serious problems. It is important to continue taking your pills on schedule. If the bleeding occurs in more than one cycle or lasts for more than a few days, talk to your doctor or health care provider.
2. Gastrointestinal Effects
The most frequent, unpleasant side effects are nausea and vomiting, stomach cramps, bloating, and a change in appetite.
3. Contact Lenses
If you wear contact lenses and notice a change in vision or an inability to wear your lenses, contact your doctor or health care provider.
4. Fluid Retention
Oral contraceptives may cause edema (fluid retention) with swelling of the fingers or ankles and may raise your blood pressure. If you experience fluid retention, contact your doctor or health care professional.
5. Melasma
A spotty darkening of the skin is possible, particularly of the face.
6. Other Side Effects
Other side effects may include change in appetite, headache, nervousness, depression, dizziness, loss of scalp hair, rash, and vaginal infections.
If any of these side effects bother you, call your doctor or health care professional.
GENERAL PRECAUTIONS
1. Missed Periods and Use of Oral Contraceptives Before or During Early Pregnancy
There may be times when you may not menstruate regularly after you have completed taking a cycle of pills. If you have taken your pills regularly and miss one menstrual period, continue taking your pills for the next cycle but be sure to inform your health care professional before doing so. If you have not taken the pills daily as instructed and missed a menstrual period, or if you missed two consecutive menstrual periods, you may be pregnant. Check with your health care professional immediately to determine whether you are pregnant. Do not continue to take oral contraceptives until you are sure you are not pregnant, but continue to use another method of contraception.
There is no conclusive evidence that oral contraceptive use is associated with an increase in birth defects, when taken inadvertently during early pregnancy. Previously, a few studies had reported that oral contraceptives might be associated with birth defects, but these studies have not been confirmed. Nevertheless, oral contraceptives or any other drugs should not be used during pregnancy unless clearly necessary and prescribed by your doctor. You should check with your doctor about risks to your unborn child of any medication taken during pregnancy.
2. While Breastfeeding
If you are breastfeeding, consult your doctor before starting oral contraceptives. Some of the drug will be passed on to the child in the milk. A few adverse effects on the child have been reported, including yellowing of the skin (jaundice) and breast enlargement. In addition, oral contraceptives may decrease the amount and quality of your milk. If possible, do not use oral contraceptives while breastfeeding. You should use another method of contraception since breastfeeding provides only partial protection from becoming pregnant and this partial protection decreases significantly as you breastfeed for longer periods of time. You should consider starting oral contraceptives only after you have weaned your child completely.
3. Laboratory Tests
If you are scheduled for any laboratory tests, tell your doctor you are taking birth control pills. Certain blood tests may be affected by birth control pills.
4. Drug Interactions
Certain drugs may interact with birth control pills to make them less effective in preventing pregnancy or cause an increase in breakthrough bleeding. Such drugs include rifampin, drugs used for epilepsy such as barbiturates (for example, phenobarbital) and phenytoin (Dilantin is one brand of this drug), phenylbutazone (Butazolidin is one brand) and possibly ampicillin and tetracyclines (several brand names). You may need to use an additional method of contraception when you take drugs which can make oral contraceptives less effective.
HOW TO TAKE THE PILL
IMPORTANT POINTS TO REMEMBER
This product (like all oral contraceptives) is intended to prevent pregnancy. It does not protect against transmission of HIV (AIDS) and other sexually transmitted diseases such as Chlamydia, genital herpes, genital warts, gonorrhea, hepatitis B, and syphilis.
BEFORE YOU START TAKING YOUR PILLS:
- BE SURE TO READ THESE DIRECTIONS:
- Before you start taking your pills.
- Anytime you are not sure what to do.
- THE RIGHT WAY TO TAKE THE PILL IS TO TAKE ONE PILL EVERY DAY AT THE SAME TIME.
- If you miss pills you could get pregnant. This includes starting the pack late. The more pills you miss, the more likely you are to get pregnant.
- MANY WOMEN HAVE SPOTTING OR LIGHT BLEEDING, OR MAY FEEL SICK TO THEIR STOMACH DURING THE FIRST 1 to 3 PACKS OF PILLS.
- If you do feel sick to your stomach, do not stop taking the pill. The problem will usually go away. If it doesn't go away, check with your doctor or clinic.
- MISSING PILLS CAN ALSO CAUSE SPOTTING OR LIGHT BLEEDING, even when you make up these missed pills.
- On the days you take 2 pills to make up for missed pills, you could also feel a little sick to your stomach.
- IF YOU HAVE VOMITING OR DIARRHEA, for any reasons, or IF YOU TAKE SOME MEDICINES, including some antibiotics, your pills may not work as well.
- Use a back-up method (such as condoms, foam, or sponge) until you check with your doctor or clinic.
- IF YOU HAVE TROUBLE REMEMBERING TO TAKE THE PILL, talk to your doctor or clinic about how to make pill-taking easier or about using another method of birth control.
- IF YOU HAVE ANY QUESTIONS OR ARE UNSURE ABOUT THE INFORMATION IN THIS LEAFLET, call your doctor or clinic.
BEFORE YOU START TAKING YOUR PILLS
- DECIDE WHAT TIME OF DAY YOU WANT TO TAKE YOUR PILL.
- It is important to take it at about the same time every day.
- LOOK AT YOUR PILL PACK TO SEE IF IT HAS 28 PILLS:
- The 28-pill pack has 21 "active" light peach pills (with hormones) to take for 3 weeks, followed by 1 week of reminder white pills (without hormones).
Refer to the sample of the wallet below.
For use of day labels, see WHEN TO START THE FIRST PACK OF PILLS
3. BE SURE YOU HAVE READY AT ALL TIMES:
ANOTHER KIND OF BIRTH CONTROL (such as condoms, foam, or sponge) to use as a back-up in case you miss pills.
An EXTRA, FULL PILL PACK.
WHEN TO START THE FIRST PACK OF PILLS
You have a choice of which day to start taking your first pack of pills. Vyfemla™ (norethindrone and ethinyl estradiol tablets USP) is available in Wallet which is designed for Sunday Start. Day 1 Start is also provided. Decide with your doctor or clinic which is the best day for you. Pick a time of day which will be easy to remember.
DAY-1 START:
- Take the first "active" light peach pill of the first pack during the first 24 hours of your period.
- You will not need to use a back-up method of birth control, since you are starting the pill at the beginning of your period.
- Take the first "active" light peach pill of the first pack on the Sunday after your period starts, even if you are still bleeding. If your period begins on Sunday, start the pack that same day.
- Use another method of birth control as a back-up method if you have sex anytime from the Sunday you start your first pack until the next Sunday (7 days). Condoms, foam, or the sponge are good back-up methods of birth control.
WHAT TO DO DURING THE MONTH
1. TAKE ONE PILL AT THE SAME TIME EVERY DAY UNTIL THE PACK IS EMPTY.
Do not skip pills even if you are spotting or bleeding between monthly periods or feel sick to your stomach (nausea).
Do not skip pills even if you do not have sex very often.
2. WHEN YOU FINISH A PACK OR SWITCH YOUR BRAND OF PILLS:
28 pills: Start the next pack on the day after your last "reminder" pill. Do not wait any days between packs.
WHAT TO DO IF YOU MISS PILLS
If you MISS 1 light peach "active" pill:
- Take it as soon as you remember. Take the next pill at your regular time. This means you may take 2 pills in 1 day.
- You do not need to use a back-up birth control method if you have sex.
If you MISS 2 light peach "active" pills in a row in WEEK 1 OR WEEK 2 of your pack:
- Take 2 pills on the day you remember and 2 pills the next day.
- Then take 1 pill a day until you finish the pack.
- You MAY BECOME PREGNANT if you have sex in the 7 days after you miss pills. You MUST use another birth control method (such as condoms, foam, or sponge) as a back-up for those 7 days.
If you MISS 2 light peach "active" pills in a row in THE 3rd WEEK:
1. If you are a Day 1 Starter:
THROW OUT the rest of the pill pack and start a new pack that same day.
If you are a Sunday Starter:
Keep taking 1 pill every day until Sunday.
On Sunday, THROW OUT the rest of the pack and start a new pack of pills that same day.
2. You may not have your period this month but this is expected. However, if you miss your period 2 months in a row, call your doctor or clinic because you might be pregnant.
3. You MAY BECOME PREGNANT if you have sex in the 7 days after you miss pills. You MUST use another birth control method (such as condoms, foam, or sponge) as a back-up for those 7 days.
If you MISS 3 OR MORE light peach "active" pills in a row (during the first 3 weeks):
1. If you are a Day 1 Starter:
THROW OUT the rest of the pill pack and start a new pack that same day.
If you are a Sunday Starter:
Keep taking 1 pill every day until Sunday.
On Sunday, THROW OUT the rest of the pack and start a new pack of pills that same day.
2. You may not have your period this month but this is expected. However, if you miss your period 2 months in a row, call your doctor or clinic because you might be pregnant.
3. You MAY BECOME PREGNANT if you have sex in the 7 days after you miss pills. You MUST use another birth control method (such as condoms, foam, or sponge) as a back-up for those 7 days.
A REMINDER FOR THOSE ON 28-DAY PACKS:
If you forget any of the 7 white "reminder" pills in Week 4:
THROW AWAY the pills you missed.
Keep taking 1 pill each day until the pack is empty. You do not need a back-up method.
FINALLY, IF YOU ARE STILL NOT SURE WHAT TO DO ABOUT THE PILLS YOU HAVE MISSED:
Use a BACK-UP METHOD anytime you have sex.
KEEP TAKING ONE “ACTIVE” PILL EACH DAY until you can reach your doctor or clinic.
-
SPL UNCLASSIFIED SECTION
1. Pregnancy Due to Pill Failure
The incidence of pill failure resulting in pregnancy is approximately 1% (i.e., one pregnancy per 100 women per year) if taken every day as directed, but more typical failure rates are about 3%. If failure does occur, the risk to the fetus is minimal.
2. Pregnancy after Stopping the Pill
There may be some delay in becoming pregnant after you stop using oral contraceptives, especially if you had irregular menstrual cycles before you used oral contraceptives. It may be advisable to postpone conception until you begin menstruating regularly once you have stopped taking the pill and desire pregnancy.
There does not appear to be any increase in birth defects in newborn babies when pregnancy occurs soon after stopping the pill.
3 Other
a. Overdosage
Serious ill effects have not been reported following ingestion of large doses of oral contraceptives by young children. Overdosage may cause nausea and withdrawal bleeding in females. In case of overdosage, contact your poison control center, health care professional, or nearest emergency room. KEEP THIS DRUG AND ALL DRUGS OUT OF THE REACH OF CHILDREN.
b. General Medical Information
Your health care professional will take a medical and family history before prescribing oral contraceptives and will examine you. The physical examination may be delayed to another time if you request it and the health care provider believes that it is a good medical practice to postpone it. You should be reexamined at least once per year. Be sure to inform your health care professional if there is a family history of any of the conditions listed previously in this leaflet. Be sure to keep all appointments with your health care professional, because this is a time to determine if there are early signs of side effects of oral contraceptive use.
Do not use the drug for any condition other than the one for which it was prescribed. This drug has been prescribed specifically for you; do not give it to others who may want birth control pills.
NONCONTRACEPTIVE EFFECTS OF ORAL CONTRACEPTIVES
In addition to preventing pregnancy, use of oral contraceptives may provide certain benefits. They are:
- Menstrual cycles may become more regular
- Blood flow during menstruation may be lighter and less iron may be lost. Therefore, anemia due to iron deficiency is less likely to occur.
- Pain or other symptoms during menstruation may be encountered less frequently
- Ectopic (tubal) pregnancy may occur less frequently
- Noncancerous cysts or lumps in the breast may occur less frequently
- Acute pelvic inflammatory disease may occur less frequently
- Oral contraceptive use may provide some protection against developing two forms of cancer: cancer of the ovaries and cancer of the lining of the uterus.
If you want more information about birth control pills, ask your doctor or pharmacist. They have a more technical leaflet called the Professional Labeling, which you may wish to read.
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL
(norethindrone and ethinyl estradiol tablets USP)
0.4 mg/0.035 mg
28 Day Regimen
Wallet Pack:
NDC: 68180-875-11
28 Tablets
(norethindrone and ethinyl estradiol tablets USP)
0.4 mg/0.035 mg
28 Day Regimen
Pouch Pack:
NDC: 68180-875-11
1 Wallet of 28 Tablets
(norethindrone and ethinyl estradiol tablets USP)
0.4 mg/0.035 mg
28 Day Regimen
Carton Pack:
NDC: 68180-875-13
3 Wallets of 28 Tablets Each
-
INGREDIENTS AND APPEARANCE
VYFEMLA
norethindrone and ethinyl estradiol kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 57297-875 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 57297-875-13 3 in 1 CARTON 12/23/2013 1 NDC: 57297-875-11 1 in 1 POUCH 1 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 21 Part 2 7 Part 1 of 2 NORETHINDRONE AND ETHINYL ESTRADIOL
norethindrone and ethinyl estradiol tabletProduct Information Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ETHINYL ESTRADIOL (UNII: 423D2T571U) (ETHINYL ESTRADIOL - UNII:423D2T571U) ETHINYL ESTRADIOL 0.035 mg NORETHINDRONE (UNII: T18F433X4S) (NORETHINDRONE - UNII:T18F433X4S) NORETHINDRONE 0.4 mg Product Characteristics Color ORANGE (light peach) Score no score Shape ROUND Size 6mm Flavor Imprint Code LU;I25 Contains Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA201886 12/23/2013 Part 2 of 2 INERT
inert tabletProduct Information Route of Administration ORAL Product Characteristics Color WHITE (white to off-white) Score no score Shape ROUND Size 6mm Flavor Imprint Code LU;U22 Contains Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA201886 12/23/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA201886 12/23/2013 Labeler - LUPIN LIMITED (675923163) Registrant - LUPIN LIMITED (675923163) Establishment Name Address ID/FEI Business Operations LUPIN LIMITED 650582310 manufacture(57297-875) , pack(57297-875)
Trademark Results [Vyfemla]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 VYFEMLA 85952227 4598385 Live/Registered |
Lupin Ltd. 2013-06-06 |
 VYFEMLA 77867291 not registered Dead/Abandoned |
Lupin Ltd. 2009-11-06 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.