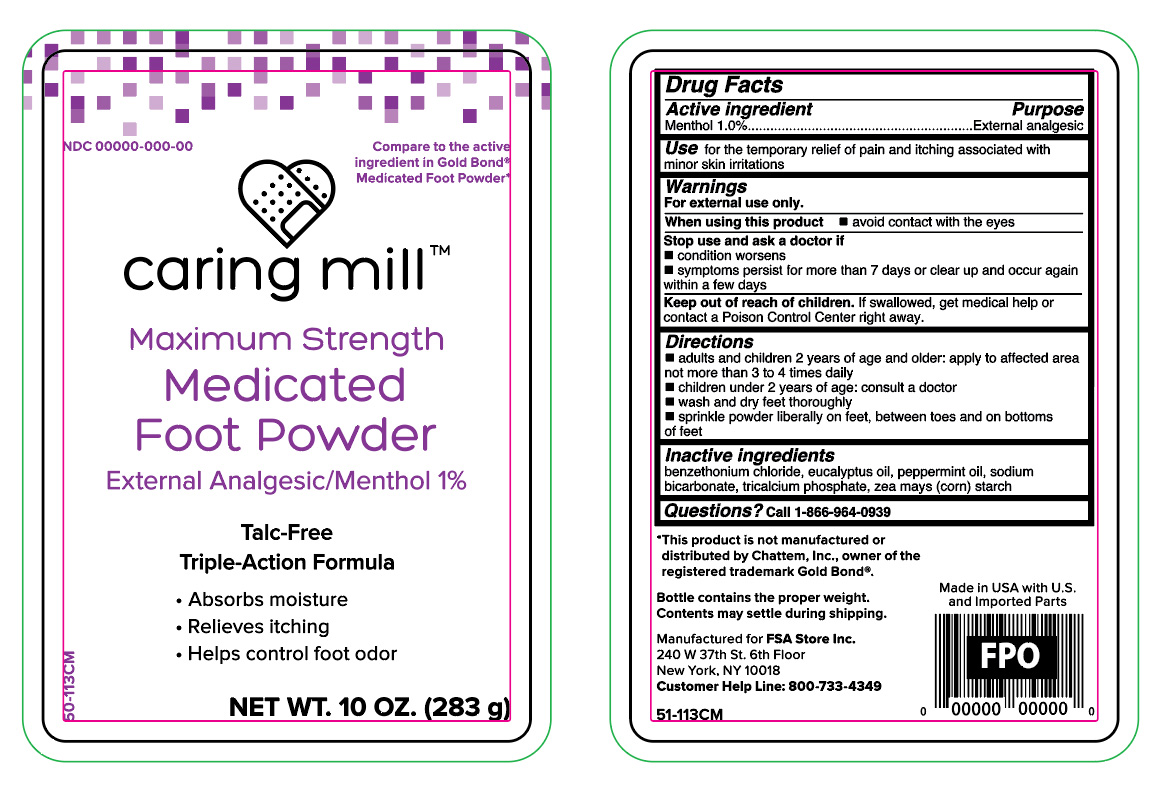
Caring Mill Medicated Foot Powder -Talc Free
Menthol by
Drug Labeling and Warnings
Menthol by is a Otc medication manufactured, distributed, or labeled by FSA Store Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
MENTHOL- maximum strength medicated foot powder talc free powder
FSA Store Inc.
----------
Caring Mill Medicated Foot Powder -Talc Free
Warnings
For external use only.
Directions
- adults and children 2 years of age and older, apply to affected area not more than 3 to 4 times daily
- children under 2 years of age, consult a doctor
- wash and dry feet thoroughly
- sprinkle powder liberally on feet, between toes and on bottoms of feet
| MENTHOL
maximum strength medicated foot powder talc free powder |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Labeler - FSA Store Inc. (049283340) |
Revised: 12/2024
Document Id: 29ba8554-c5f1-4a05-e063-6394a90a520e
Set id: fabc23eb-7750-1b5a-e053-6294a90aed03
Version: 2
Effective Time: 20241220
Trademark Results [Menthol]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MENTHOL 77935694 3835052 Dead/Cancelled |
Reynolds Innovations Inc. 2010-02-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.