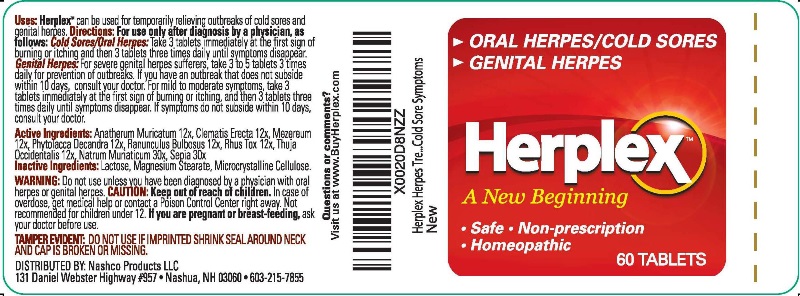
HERPLEX- anatherum muricatum, clematis erecta, mezereum, phytolacca decandra, ranunculus bulbosus, rhus tox, thuja occidentalis, natrum muriaticum, sepia tablet
Herplex by
Drug Labeling and Warnings
Herplex by is a Homeopathic medication manufactured, distributed, or labeled by Nashco Products LLC, Apotheca Company. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENTS:
- USES:
-
WARNINGS:
Do not use unless you have been diagnosed by a physician with oral herpes or genital herpes.
CAUTION: Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away. Not recommended for children under 12.
If you are pregnant or breast-feeding, ask your doctor before use.
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SHRINK SEAL AROUND NECK AND CAP IS BROKEN OR MISSING.
- KEEP OUT OF REACH OF CHILDREN:
-
DIRECTIONS:
For use only after diagnoses by a physician, as follows:
Cold Sores/Oral Herpes: Take 3 tablets immediately at the first sign of burning or itching and then 3 tablets three times daily until symptoms disappear.
Genital Herpes: For severe genital herpes sufferers, take 3 to 5 tablets 3 times daily for prevention of outbreaks. If you have an outbreak that does not subside within 10 days, consult your doctor. For mild to moderate symptoms, take 3 tablets immediately at the first sign of burning or itching, and then 3 tablets three times daily until symptoms disappear. If symptoms do not subside within 10 days, consult your doctor.
- USES:
- INACTIVE INGREDIENTS:
- QUESTIONS OR COMMENTS:
- PACKAGE LABEL DISPLAY:
-
INGREDIENTS AND APPEARANCE
HERPLEX
anatherum muricatum, clematis erecta, mezereum, phytolacca decandra, ranunculus bulbosus, rhus tox, thuja occidentalis, natrum muriaticum, sepia tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 72968-0002 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHRYSOPOGON ZIZANIOIDES ROOT (UNII: 37TB8LUP9Z) (CHRYSOPOGON ZIZANIOIDES ROOT - UNII:37TB8LUP9Z) CHRYSOPOGON ZIZANIOIDES ROOT 12 [hp_X] CLEMATIS RECTA FLOWERING TOP (UNII: 396421SP9F) (CLEMATIS RECTA FLOWERING TOP - UNII:396421SP9F) CLEMATIS RECTA FLOWERING TOP 12 [hp_X] DAPHNE MEZEREUM BARK (UNII: X2N6E405GV) (DAPHNE MEZEREUM BARK - UNII:X2N6E405GV) DAPHNE MEZEREUM BARK 12 [hp_X] PHYTOLACCA AMERICANA ROOT (UNII: 11E6VI8VEG) (PHYTOLACCA AMERICANA ROOT - UNII:11E6VI8VEG) PHYTOLACCA AMERICANA ROOT 12 [hp_X] RANUNCULUS BULBOSUS (UNII: AEQ8NXJ0MB) (RANUNCULUS BULBOSUS - UNII:AEQ8NXJ0MB) RANUNCULUS BULBOSUS 12 [hp_X] TOXICODENDRON PUBESCENS LEAF (UNII: 6IO182RP7A) (TOXICODENDRON PUBESCENS LEAF - UNII:6IO182RP7A) TOXICODENDRON PUBESCENS LEAF 12 [hp_X] THUJA OCCIDENTALIS LEAFY TWIG (UNII: 1NT28V9397) (THUJA OCCIDENTALIS LEAFY TWIG - UNII:1NT28V9397) THUJA OCCIDENTALIS LEAFY TWIG 12 [hp_X] SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 30 [hp_X] SEPIA OFFICINALIS JUICE (UNII: QDL83WN8C2) (SEPIA OFFICINALIS JUICE - UNII:QDL83WN8C2) SEPIA OFFICINALIS JUICE 30 [hp_X] Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) Product Characteristics Color white Score no score Shape ROUND (convex) Size 6mm Flavor Imprint Code diamond Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72968-0002-1 60 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 04/18/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 04/18/2019 Labeler - Nashco Products LLC (116986692) Registrant - Apotheca Company (844330915) Establishment Name Address ID/FEI Business Operations Apotheca Company 844330915 manufacture(72968-0002) , api manufacture(72968-0002) , label(72968-0002) , pack(72968-0002)
Trademark Results [Herplex]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 HERPLEX 78643504 3228758 Live/Registered |
ASCENT IP HOLDINGS, LLC 2005-06-03 |
 HERPLEX 74050747 1633669 Dead/Cancelled |
Allergan, Inc. 1990-04-19 |
 HERPLEX 73445368 1295622 Dead/Cancelled |
Allergan Pharmaceuticals, Inc. 1983-09-26 |
 HERPLEX 72146554 0747506 Dead/Expired |
ALLERGAN PHARMACEUTICALS 1962-06-11 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.