AGAMREE by Catalyst Pharmaceuticals, Inc. AGAMREE- vamorolone kit
AGAMREE by
Drug Labeling and Warnings
AGAMREE by is a Prescription medication manufactured, distributed, or labeled by Catalyst Pharmaceuticals, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use AGAMREE ®safely and effectively. See full prescribing information for AGAMREE.
AGAMREE (vamorolone) oral suspension
Initial U.S. Approval: 2023RECENT MAJOR CHANGES
Warnings and Precautions, Immunosuppression and Increased Risk of Infection ( 5.2) 06/2024 INDICATIONS AND USAGE
AGAMREE is a corticosteroid indicated for the treatment of Duchenne muscular dystrophy (DMD) in patients 2 years of age and older. ( 1)
DOSAGE AND ADMINISTRATION
- The recommended dosage is 6 mg/kg taken orally once daily preferably with a meal, up to a maximum daily dosage of 300 mg for patients weighing more than 50 kg. ( 2.2)
- In patients with mild to moderate hepatic impairment, the recommended dosage is 2 mg/kg taken orally once daily preferably with a meal, up to a maximum daily dosage of 100 mg for patients weighing more than 50 kg. ( 2.3)
- Decrease dosage gradually when administered for more than one week. ( 2.7)
DOSAGE FORMS AND STRENGTHS
Oral Suspension: 40 mg/mL ( 3)
CONTRAINDICATIONS
Hypersensitivity to vamorolone or any of the inactive ingredients in AGAMREE ( 4)
WARNINGS AND PRECAUTIONS
- Alterations in Endocrine Function:Hypothalamic-pituitary-adrenal axis suppression, cushingoid features, and hyperglycemia can occur. Monitor patients for these conditions with chronic use of AGAMREE. ( 2.7, 5.1)
- Immunosuppression and Increased Risk of Infection:Increased risk of new infections, exacerbation, dissemination, or reactivation of latent infections, which can be severe and at times fatal; signs and symptoms of infections may be masked. ( 5.2)
- Alterations in Cardiovascular/Renal Function:Monitor for elevated blood pressure and monitor sodium and potassium levels in patients chronically treated with AGAMREE. ( 5.3)
- Gastrointestinal Perforation:Increased risk in patients with certain GI disorders; signs and symptoms may be masked. ( 5.4)
- Behavioral and Mood Disturbances:May include euphoria, insomnia, mood swings, personality changes, severe depression, and psychosis. ( 5.5)
- Effects on Bones:Monitor for decreases in bone mineral density with chronic use of AGAMREE. ( 5.6)
- Ophthalmic Effects:May include cataracts, infections, and glaucoma; monitor intraocular pressure in patients chronically treated with AGAMREE. ( 5.7)
- Vaccination:Do not administer live or live attenuated vaccines to patients receiving immunosuppressive doses of corticosteroids. Administer live-attenuated or live vaccines at least 4 to 6 weeks prior to starting AGAMREE. ( 5.8)
ADVERSE REACTIONS
The most common adverse reactions (>10% for AGAMREE and greater than placebo) are cushingoid features, psychiatric disorders, vomiting, weight increased, and vitamin D deficiency. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Catalyst Pharmaceuticals, Inc. at 1-844-347-3277 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 6/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Assessments Prior to First Dose of AGAMREE
2.2 Dosing Information
2.3 Recommended Dosage for Hepatic Impairment
2.4 Important Preparation and Administration Instructions
2.5 Switching from Corticosteroid Treatment to AGAMREE
2.6 Dosage Modification for Use with Strong CYP3A4 Inhibitors
2.7 Discontinuation
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Alterations in Endocrine Function
5.2 Immunosuppression and Increased Risk of Infection
5.3 Alterations in Cardiovascular/Renal Function
5.4 Gastrointestinal Perforation
5.5 Behavioral and Mood Disturbances
5.6 Effects on Bones
5.7 Ophthalmic Effects
5.8 Immunizations
5.9 Effects on Growth and Development
5.10 Myopathy
5.11 Kaposi's Sarcoma
5.12 Thromboembolic Events
5.13 Anaphylaxis
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on Vamorolone
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Assessments Prior to First Dose of AGAMREE
Administer all immunizations according to immunization guidelines prior to starting AGAMREE. Administer live-attenuated or live vaccines at least 4 to 6 weeks prior to starting AGAMREE [see Warnings and Precautions ( 5.8)].
2.2 Dosing Information
The recommended dosage of AGAMREE is 6 mg/kg taken orally once daily preferably with a meal, up to a maximum daily dosage of 300 mg for patients weighing more than 50 kg.
Some patients may respond to a dose of 2 mg/kg daily. Doses may be titrated down to 2 mg/kg/day as needed, based on individual tolerability.
2.3 Recommended Dosage for Hepatic Impairment
The recommended dosage of AGAMREE in patients with mild (Child-Pugh A) to moderate (Child-Pugh B) hepatic impairment is 2 mg/kg taken orally once daily preferably with a meal, up to a maximum daily dosage of 100 mg for patients weighing more than 50 kg [see Use in Specific Populations ( 8.6) and Clinical Pharmacology ( 12.3)] .
Doses may be titrated down based on individual tolerability.
2.4 Important Preparation and Administration Instructions
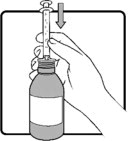
Shake AGAMREE oral suspension well for about 30 seconds before administration.
Use only the oral syringe provided with the product. After withdrawing the appropriate dose into the oral syringe, dispense directly into the mouth.
Discard any unused AGAMREE oral suspension remaining after 3 months of first opening the bottle.
2.5 Switching from Corticosteroid Treatment to AGAMREE
Patients can be switched from oral corticosteroid treatment (such as prednisone or deflazacort) to AGAMREE without treatment interruption or period of prior corticosteroid dosage reduction to minimize the risk for adrenal insufficiency.
Patients switching after long-term treatment with oral corticosteroids should start AGAMREE at a dosage of 6 mg/kg/day.
2.6 Dosage Modification for Use with Strong CYP3A4 Inhibitors
The recommended dosage of AGAMREE when administered with strong CYP3A4 inhibitors is 4 mg/kg taken orally once daily preferably with a meal, up to a maximum daily dosage of 200 mg for patients weighing more than 50 kg [see Drug Interactions ( 7.1) and Clinical Pharmacology ( 12.3)].
Doses may be titrated down based on individual tolerability.
2.7 Discontinuation
Dosage of AGAMREE must be decreased gradually if the drug has been administered for more than one week [see Warnings and Precautions ( 5.1)].
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
AGAMREE is contraindicated in patients with known hypersensitivity to vamorolone or to any of the inactive ingredients of AGAMREE. Instances of hypersensitivity, including anaphylaxis, have occurred in patients receiving corticosteroid therapy [see Warnings and Precautions ( 5.13)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Alterations in Endocrine Function
Corticosteroids, such as AGAMREE, can cause serious and life-threatening alterations in endocrine function, especially with chronic use. Monitor patients receiving AGAMREE for Cushing's syndrome, hyperglycemia, and adrenal insufficiency after AGAMREE withdrawal. In addition, patients with hypopituitarism, primary adrenal insufficiency or congenital adrenal hyperplasia, altered thyroid function, or pheochromocytoma may be at increased risk for adverse endocrine events.
Risk of Adrenal Insufficiency Following Withdrawal
AGAMREE produces reversible hypothalamic-pituitary-adrenal (HPA) axis suppression, with the potential for the development of secondary adrenal insufficiency after withdrawal. Acute adrenal insufficiency can occur if AGAMREE is withdrawn abruptly, and could be fatal. The degree and duration of adrenocortical insufficiency produced is variable among patients and depends on the dose and duration of therapy.
The risk of adrenal insufficiency is reduced by gradually tapering the dose when withdrawing treatment. The insufficiency may persist, however, for months after discontinuation of prolonged therapy; therefore, in any situation of stress occurring during that period of discontinuation, supplementation with a systemic corticosteroid is recommended. For patients already taking corticosteroids during times of stress, the dosage may need to be increased.
A steroid “withdrawal syndrome”, seemingly unrelated to adrenocortical insufficiency, may also occur following abrupt discontinuance of corticosteroids. This syndrome includes symptoms such as anorexia, nausea, vomiting, lethargy, headache, fever, joint pain, desquamation, myalgia, and/or weight loss. These effects are thought to be due to the sudden change in corticosteroid concentration rather than too low corticosteroid levels.
Cushing's Syndrome
Cushing's syndrome (hypercortisolism) occurs with prolonged exposure to exogenous corticosteroids, including AGAMREE, and symptoms include hypertension, truncal obesity and thinning of the limbs, purple striae, facial rounding, facial plethora, muscle weakness, easy and frequent bruising with thin fragile skin, posterior neck fat deposition, osteopenia, acne, amenorrhea, hirsutism, and psychiatric abnormalities.
Hyperglycemia
Corticosteroids can increase blood glucose, worsen pre-existing diabetes, predispose those on long-term therapy to diabetes mellitus, and may reduce the effect of anti-diabetic drugs.
Monitor blood glucose at regular intervals in patients treated with AGAMREE. For patients with hyperglycemia, anti-diabetic treatment should be initiated or adjusted accordingly.
Considerations for Use in Patients with Altered Thyroid Function
Metabolic clearance of corticosteroids is decreased in hypothyroid patients and increased in hyperthyroid patients. Changes in thyroid status of the patient may necessitate a dose adjustment of the corticosteroid. When concomitant administration of AGAMREE and levothyroxine is required, administration of AGAMREE should precede the initiation of levothyroxine therapy to reduce the risk of adrenal crisis.
5.2 Immunosuppression and Increased Risk of Infection
Corticosteroids, including AGAMREE, suppress the immune system and increase the risk of infection with any pathogen, including viral, bacterial, fungal, protozoan, or helminthic pathogens.
Corticosteroids can:
- reduce resistance to new infections
- exacerbate existing infections
- increase the risk of disseminated infections
- increase the risk of reactivation or exacerbation of latent infections
- mask some signs of infection
Corticosteroid-associated infections can be mild but can be severe, and at times fatal. The rate of infectious complications increases with increasing corticosteroid dosages.
Monitor for the development of infection and consider AGAMREE withdrawal or dosage reduction as needed.
Tuberculosis
If AGAMREE is used to treat a condition in patients with latent tuberculosis or tuberculin reactivity, reactivation of tuberculosis may occur. Closely monitor such patients for reactivation. During prolonged AGAMREE therapy, patients with latent tuberculosis or tuberculin reactivity should receive chemoprophylaxis.
Varicella Zoster and Measles Viral Infections
Varicella and measles can have a serious or even fatal course in non-immune patients taking corticosteroids, including AGAMREE. In corticosteroid-treated patients who have not had these diseases or are non-immune, particular care should be taken to avoid exposure to varicella and measles.
- If an AGAMREE-treated patient is exposed to varicella, prophylaxis with varicella zoster immunoglobulin may be indicated. If varicella develops, treatment with antiviral agents may be considered.
- If an AGAMREE-treated patient is exposed to measles, prophylaxis with immunoglobulin may be indicated.
Hepatitis B Virus Reactivation
Hepatitis B virus reactivation can occur in patients who are hepatitis B carriers treated with immunosuppressive dosages of corticosteroids, including AGAMREE. Reactivation can also occur infrequently in corticosteroid-treated patients who appear to have resolved hepatitis B infection.
Screen patients for hepatitis B infection before initiating immunosuppressive (e.g., prolonged) treatment with AGAMREE. For patients who show evidence of hepatitis B infection, recommend consultation with physicians with expertise in managing hepatitis B regarding monitoring and consideration for hepatitis B antiviral therapy.
Fungal Infections
Corticosteroids, including AGAMREE, may exacerbate systemic fungal infections; therefore, avoid AGAMREE use in the presence of such infections unless AGAMREE is needed to control drug reactions. For patients on chronic AGAMREE therapy who develop systemic fungal infections, AGAMREE withdrawal or dosage reduction is recommended.
Amebiasis
Corticosteroids, including AGAMREE, may activate latent amebiasis. Therefore, it is recommended that latent amebiasis or active amebiasis be ruled out before initiating AGAMREE in any patients who have spent time in the tropics or patients with unexplained diarrhea.
Strongyloides Infestation
Corticosteroids, including AGAMREE, should be used with great care in patients with known or suspected Strongyloides (threadworm) infestation. In such patients, corticosteroid-induced immunosuppression may lead to Strongyloides hyperinfection and dissemination with widespread larval migration, often accompanied by severe enterocolitis and potentially fatal gram-negative septicemia.
5.3 Alterations in Cardiovascular/Renal Function
Corticosteroids, including AGAMREE, can cause elevation of blood pressure, salt and water retention, and increased excretion of potassium and calcium.
Monitor blood pressure and assess for signs and symptoms of volume overload. Monitor serum potassium levels.
AGAMREE should be used with caution in patients with congestive heart failure, hypertension, or renal insufficiency. Literature reports suggest an association between use of corticosteroids and left free wall rupture after a recent myocardial infarction; therefore, therapy with AGAMREE should be used with great caution in these patients.
5.4 Gastrointestinal Perforation
There is an increased risk of gastrointestinal perforation with use of corticosteroids in patients with certain gastrointestinal disorders, such as active or latent peptic ulcers, diverticulitis, fresh intestinal anastomoses, and non-specific ulcerative colitis. Signs of gastrointestinal perforation, such as peritoneal irritation, may be masked in patients receiving corticosteroids.
Avoid AGAMREE if there is a probability of impending perforation, abscess, or other pyogenic infections; diverticulitis; fresh intestinal anastomoses; or active or latent peptic ulcer.
5.5 Behavioral and Mood Disturbances
Potentially severe psychiatric adverse reactions may occur with systemic corticosteroids, including AGAMREE. Symptoms typically emerge within a few days or weeks of starting treatment and may be dose-related. These reactions may improve after either dose reduction or withdrawal, although pharmacologic treatment may be necessary.
In Study 1, psychiatric adverse reactions were reported in 21% of patients on AGAMREE 6 mg/kg, 10% of patients on AGAMREE 2 mg/kg, and 14% of patients on placebo. Psychiatric adverse reactions reported on AGAMREE resolved without requiring treatment or drug discontinuation.
In adults, psychiatric adverse reactions with corticosteroids usually involve hypomanic or manic symptoms (e.g., euphoria, insomnia, mood swings) during treatment and depressive episodes after discontinuation of treatment. In children receiving corticosteroids, psychiatric adverse reactions usually involve hyperactivity symptoms (e.g., irritability, aggressive behavior, increased frequency of tantrums, and mood swings) and sleep disorder during treatment. Inform patients or caregivers of the potential for behavioral and mood changes and encourage them to seek medical attention if psychiatric symptoms develop, especially if depressed mood or suicidal ideation is suspected.
5.6 Effects on Bones
Decreased Bone Mineral Density
Corticosteroids, such as AGAMREE, decrease bone formation and increase bone resorption both through their effect on calcium regulation (i.e., decreasing absorption and increasing excretion) and inhibition of osteoblast function. This, together with a decrease in the protein matrix of the bone secondary to an increase in protein catabolism and reduced sex hormone production, may lead to inhibition of bone growth in pediatric patients and the development of bone loss at any age. Bone loss can predispose patients to vertebral and long bone fractures.
Consider a patient's risk of osteoporosis before initiating corticosteroid therapy. Monitor bone mineral density in patients on long-term treatment with AGAMREE.
5.7 Ophthalmic Effects
The use of corticosteroids, such as AGAMREE, may produce posterior subcapsular cataracts. Corticosteroids may also cause glaucoma with possible damage to the optic nerves, and may increase the risk of secondary ocular infections caused by bacteria, fungi, or viruses. Corticosteroids are not recommended for patients with active ocular herpes simplex. Intraocular pressure may become elevated in some patients taking corticosteroids. If treatment with AGAMREE is continued for more than 6 weeks, monitor intraocular pressure.
5.8 Immunizations
Administer all immunizations according to immunization guidelines prior to starting AGAMREE. Administer live-attenuated or live vaccines at least 4 to 6 weeks prior to starting AGAMREE. Patients on AGAMREE may receive concurrent vaccinations, except for live-attenuated or live vaccines.
5.9 Effects on Growth and Development
Long-term use of corticosteroids, including AGAMREE, can have negative effects on growth and development in children.
5.10 Myopathy
Patients receiving corticosteroids and concomitant therapy with neuromuscular blocking agents (e.g., pancuronium) or patients with disorders of neuromuscular transmission (e.g., myasthenia gravis) may be at increased risk of developing acute myopathy. This acute myopathy is generalized, may involve ocular and respiratory muscles, and may result in quadriparesis. Clinical improvement or recovery after stopping corticosteroids may require weeks to years.
5.11 Kaposi's Sarcoma
Kaposi's sarcoma has been reported to occur in patients receiving corticosteroid therapy, most often for chronic conditions. Discontinuation of corticosteroids may result in clinical improvement of Kaposi's sarcoma.
5.12 Thromboembolic Events
Observational studies have shown an increased risk of thromboembolism (including venous thromboembolism) particularly with higher cumulative doses of corticosteroids. It is unclear if risk differs by daily dose or duration of use. Use AGAMREE with caution in patients who have or may be predisposed to thromboembolic disorders.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed in more detail in other sections:
- Alterations in Endocrine Function [see Warnings and Precautions ( 5.1)]
- Immunosuppression and Increased Risk of Infection [see Warnings and Precautions ( 5.2)]
- Alterations in Cardiovascular/Renal Function [see Warnings and Precautions ( 5.3)]
- Gastrointestinal Perforation [see Warnings and Precautions ( 5.4)]
- Behavioral and Mood Disturbances [see Warnings and Precautions ( 5.5)]
- Effects on Bones [see Warnings and Precautions ( 5.6)]
- Ophthalmic Effects [see Warnings and Precautions ( 5.7)]
- Immunizations [see Warnings and Precautions ( 5.8)]
- Effects on Growth and Development [see Warnings and Precautions ( 5.9)]
- Myopathy [see Warnings and Precautions ( 5.10)]
- Kaposi's Sarcoma [see Warnings and Precautions ( 5.11)]
- Thromboembolic Events [see Warnings and Precautions ( 5.12)]
- Anaphylaxis [see Warnings and Precautions ( 5.13)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Common Adverse Reactions in Clinical Studies
Table 1lists the adverse reactions that occurred in ≥ 5% of the patients treated with AGAMREE 6 mg/kg/day (N=28) or AGAMREE 2 mg/kg/day (N=30) and that occurred more frequently than in the patients who received placebo (N=29) in Study 1 [see Clinical Studies ( 14)] , which was 24 weeks and included patients with DMD between the ages of 4 and 7 years.
Table 1: Adverse Reactions in Patients with DMD that Occurred in ≥ 5% of Patients Treated with AGAMREE and More Frequently than in Patients Who Received Placebo During 24 Weeks (Study 1) 1Includes the following adverse reactions that occurred more frequently in the AGAMREE group than in placebo: abnormal behavior, aggression, agitation, anxiety, irritability, mood altered, sleep disorder, and stereotypy.
Adverse Reaction AGAMREE
2 mg/kg/d (N=30)
%AGAMREE
6 mg/kg/d (N=28)
%Placebo
(N=29)
%Cushingoid Features 7 29 0 Psychiatric disorders 1 7 21 14 Vomiting 17 14 7 Weight increased 0 11 3 Vitamin D deficiency 7 11 0 Cough 10 7 3 Headache 7 7 3 Diarrhea 3 7 3 Increased appetite 3 7 3 Rhinitis 3 7 3 In a separate open-label safety study of pediatric patients aged 2 to less than 4 years (n=16) and pediatric patients aged 7 to less than 18 years (n=16) with DMD, adverse reactions were similar to those seen in the Study 1 pediatric patients.
-
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on Vamorolone
Co-administration of AGAMREE with itraconazole, a strong CYP3A4 inhibitor, increases vamorolone exposure [see Clinical Pharmacology ( 12.3)] . Reduce the dosage of AGAMREE in patients when strong CYP3A4 inhibitors are used concomitantly [see Dosage and Administration ( 2.6)] . No dosage adjustments are required when AGAMREE is concomitantly administered with moderate or weak CYP3A4 inhibitors.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
AGAMREE is indicated for use for the treatment of DMD, which is a disease of young male patients. However, corticosteroids in general should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Infants born to mothers who have received substantial doses of corticosteroids during pregnancy should be carefully observed for signs of hypoadrenalism. There are no data on the use of AGAMREE during pregnancy.
Adverse developmental outcomes, including orofacial clefts (cleft lip, with or without cleft palate) and intrauterine growth restriction, and decreased birth weight, have been reported with maternal use of corticosteroids during pregnancy. Some epidemiologic studies report an increased risk of orofacial clefts from about 1 per 1000 infants to 3 to 5 per 1000 infants; however, a risk for orofacial clefts has not been observed in all clinical studies. Intrauterine growth restriction and decreased birth weight appear to be dose-related; however, the underlying maternal condition may also contribute to these risks (see Clinical Considerationsand Data) .
Animal reproduction studies have not been conducted with AGAMREE.
Animal reproduction studies conducted with corticosteroids in pregnant mice, rats, hamsters, and rabbits using clinically relevant doses have shown an increased incidence of cleft palate. An increase in embryofetal death, intrauterine growth retardation, and constriction of the ductus arteriosus were observed in some animal species.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
Hypoadrenalism may occur in infants born to mothers receiving corticosteroids during pregnancy. Infants should be carefully observed for signs of hypoadrenalism, such as poor feeding, irritability, weakness, and vomiting, and managed accordingly [see Warnings and Precautions ( 5.1)].
Data
Human Data
Multiple cohort and case-controlled studies in humans suggest that maternal corticosteroid use during the first trimester increases the rate of cleft lip, with or without cleft palate, from about 1/1000 infants to 3-5/1000 infants. Two prospective case-controlled studies showed decreased birth weight in infants exposed to maternal corticosteroids in utero.
8.2 Lactation
Risk Summary
There are no data on the presence of vamorolone in human milk or the effects on milk production.
AGAMREE is indicated for use for the treatment of DMD, which is a disease of young male patients. However, systemically administered corticosteroids appear in human milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need and any potential adverse effects on the breastfed infant.
8.4 Pediatric Use
The safety and effectiveness of AGAMREE for the treatment of DMD have been established in patients 2 years of age and older. Use of AGAMREE in pediatric patients is supported by a multicenter, randomized, double-blind, placebo- and active-controlled study in 121 males 4 to less than 7 years of age [see Clinical Studies ( 14)]. Use of AGAMREE in patients 2 years to less than 4 years of age and 7 to less than 18 years of age is supported by findings of efficacy and safety in patients 4 to less than 7 years of age with DMD, and by pharmacokinetic and safety data from patients 2 to 4 years of age and 7 to less than 18 years of age [see Adverse Reactions ( 6.1) and Clinical Pharmacology ( 12.3)] .
The safety and effectiveness in pediatric patients below the age of 2 years have not been established.
Juvenile Animal Toxicity Data
Oral administration of vamorolone (0, 15, 30, or 100 mg/kg/day) to juvenile mice from postnatal days 21 to 81 resulted in no adverse effects on neurobehavioral function, sexual maturation, or reproductive function. Atrophy of the adrenal cortex, degeneration/necrosis of the liver, and decreased lymphocytes in lymphatic tissues were observed at all doses. A no-effect dose for general toxicity was not identified. Plasma exposures (AUC) at the lowest dose tested (15 mg/kg/day) were lower than that in humans at the maximum recommended human dose (300 mg/day).
8.5 Geriatric Use
DMD is largely a disease of children and young adults; therefore, there is no geriatric experience with AGAMREE.
8.6 Hepatic Impairment
Moderate hepatic impairment increases vamorolone exposure [see Clinical Pharmacology ( 12.3)]. Reduce the AGAMREE dosage in patients with mild to moderate hepatic impairment [see Dosage and Administration ( 2.3)]. There is no clinical experience with AGAMREE in patients with severe hepatic impairment, and a dosing recommendation cannot be provided for patients with severe hepatic impairment.
- 10 OVERDOSAGE
-
11 DESCRIPTION
AGAMREE (vamorolone) oral suspension contains vamorolone, a corticosteroid. Vamorolone [17α,21-dihydroxy-16α-methyl-pregna-1,4,9(11)-triene-3,20-dione] is a white to off-white powder with a molecular formula of C 22H 28O 4and a molecular weight of 356.46 g/mol. Its structural formula is:
Vamorolone is freely soluble in methanol and dioxane and sparingly soluble in ethanol and acetone.
AGAMREE for oral administration is available as an oral suspension in a strength of 40 mg/mL. The oral suspension contains vamorolone and the following inactive ingredients: citric acid (monohydrate), disodium phosphate, glycerin, hydrochloric acid (for pH adjustment), orange flavor, sodium benzoate, sucralose, water, and xanthan gum.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Vamorolone is a corticosteroid that acts through the glucocorticoid receptor to exert anti-inflammatory and immunosuppressive effects. The precise mechanism by which vamorolone exerts its effect in patients with DMD is unknown.
12.2 Pharmacodynamics
Vamorolone produced a dose-dependent decrease in morning cortisol levels in the clinical studies. Treatment with corticosteroids is associated with a suppression of endogenous cortisol concentrations and an impairment of the hypothalamus-pituitary-adrenal (HPA) axis function. A dose-dependent increase in leukocyte counts and lymphocyte counts was observed in clinical studies with vamorolone.
12.3 Pharmacokinetics
The major route of elimination is by metabolism with subsequent excretion of metabolites into urine. The pharmacokinetics (PK) are linear and vamorolone exposure increases proportionally with either single (0.1 to 20 mg/kg) or multiple (0.25 to 20 mg/kg) doses. Vamorolone does not accumulate with repeated administration after once daily dosing.
Absorption
After oral administration with food, the median T maxis about 2 hours (range 0.5 to 5 hours).
Effect of Food
Co-administration of vamorolone (2 mg/kg) with a high-fat/high-calorie meal reduced C maxby 18%, increased AUC by 13%, and delayed T maxby one hour. Co-administration of vamorolone (2 mg/kg) with a low-fat/low-calorie meal reduced C maxby 4%, increased AUC by 14%, and delayed T maxby one hour [see Dosage and Administration ( 2.2)].
Distribution
The apparent volume of distribution of vamorolone for a DMD patient with a body weight of 20 kg taking AGAMREE with a meal is 162 L based on the population PK analysis. Protein binding is 88.1% in vitro. The blood to plasma ratio is approximately 0.87.
Elimination
Vamorolone clearance for a DMD patient with a body weight of 20 kg taking AGAMREE with a meal is 58 L/h based on the population PK analysis. The terminal elimination half-life of vamorolone is approximately 2 hours.
Metabolism
Vamorolone is metabolized via multiple Phase I and Phase II metabolic pathways, such as glucuronidation, hydroxylation, and reduction. The main plasma and urine metabolites are formed through direct glucuronidation as well as hydrogenation with subsequent glucuronidation. Metabolism of vamorolone is mediated through CYP3A4/5, CYP2C8, UGT1A3, UGT2B7, and UGT2B17.
Specific Populations
No clinically significant differences in the pharmacokinetics of vamorolone were observed based on race and sex.
Pediatric Patients
In children ages 4 to 7 years (N=12) who were administered 6 mg/kg of AGAMREE daily, on Day 1 and Day 14, the C maxvalues (arithmetic mean, SD) of vamorolone were 856 ng/mL (471) and 970 ng/mL (270), respectively, and the AUC 24values (arithmetic mean, SD) of vamorolone were 3279 ng·h/mL (1693) and 3606 ng·h/mL (897), respectively. The pharmacokinetics of vamorolone was also characterized in DMD children ages 2 to 4 (N=6). Similar PK parameters were observed in younger children after administration of 6 mg/kg AGAMREE when compared to older children.
Patients with Hepatic Impairment
In a clinical study (N=16), vamorolone C maxand AUC 0infvalues were increased by approximately 1.7- and 2.6-fold, respectively, in subjects with moderate hepatic impairment (Child-Pugh Class B) compared with healthy matched controls [see Dosage and Administration ( 2.3) and Use in Specific Populations ( 8.6)] . There is no experience with vamorolone in patients with severe hepatic impairment.
Drug Interaction Studies
Effect of Strong CYP3A4 Inhibitors on Vamorolone
Compared to administration of vamorolone alone, administration of vamorolone following multiple doses of a strong CYP3A4 inhibitor (itraconazole) increased vamorolone C maxand AUC by 8% and 44%, respectively [see Drug Interactions ( 7.1)]. There is no experience with vamorolone when coadministered with moderate and weak CYP3A4 inhibitors.
In Vitro Studies
Metabolizing Enzymes
Vamorolone induces CYP3A4 in vitro. Other compounds that are substrates of CYP3A4 may have decreased plasma concentrations when co-administered with AGAMREE. However, no clinical drug interaction studies with CYP3A4 substrates were conducted.
Vamorolone does not inhibit CYP or UGT isoenzymes at clinically relevant concentrations.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Mutagenesis
Vamorolone was negative for genotoxicity in in vitro (bacterial reverse mutation and cultured mouse lymphocyte chromosomal aberration) and in vivo (mouse micronucleus) assays.
Impairment of Fertility
Fertility studies in animals were not conducted with vamorolone. Oral administration of vamorolone (0, 2, 10, or 50 mg/kg/day) for 39 weeks to dogs resulted in spermatocyte and spermatid degeneration in the testes and oligospermia and germ cell debris in the epididymis in males at the high dose and absence of corpora lutea in the ovaries in females at all doses. Plasma exposures (AUC) in males at the no-effect dose for testicular toxicity (10 mg/kg) was lower than that at the maximum recommended human dose of AGAMREE (300 mg/day).
-
14 CLINICAL STUDIES
The effectiveness of AGAMREE for the treatment of Duchenne muscular dystrophy (DMD) was evaluated in a multicenter, randomized, double-blind, parallel-group, placebo- and active-controlled, multinational 24-week study (Study 1; NCT03439670). The study randomized 121 male patients with DMD to one of the following treatment groups: AGAMREE 6 mg/kg/day (n=30), AGAMREE 2 mg/kg/day (n=30), prednisone 0.75 mg/kg/day (n=31), or placebo (n=30) for 24 weeks. After 24 weeks, patients on prednisone and placebo received either AGAMREE 6 mg/kg/day (n=29) or AGAMREE 2 mg/kg/day (n=29) for an additional 20 weeks. The study included patients 4 to less than 7 years of age at time of enrollment in the study who were corticosteroid naïve and ambulatory, with a confirmed diagnosis of DMD. At baseline, patients had a mean age of 5.4 years, 83% were Caucasian, 10% were Asian, and 96% were not Hispanic or Latino.
The primary endpoint was the change from baseline to Week 24 in Time to Stand Test (TTSTAND) velocity for AGAMREE 6 mg/kg/day compared to placebo. TTSTAND velocity is a measure of muscle function that measures the time required for the patient to stand to an erect position from a supine position (floor). The key secondary endpoints consisted of change from baseline to Week 24 in TTSTAND velocity (AGAMREE 2 mg/kg/day vs placebo), 6 Minute Walk Test (6MWT) distance (AGAMREE 6 mg/kg/day vs placebo and 2 mg/kg/day vs placebo) and Time to Run/Walk 10 meters (TTRW) velocity (AGAMREE 6 mg/kg/day vs placebo and 2 mg/kg/day vs placebo). The 6MWT measures the distance that a patient can walk on a flat, hard surface in a period of 6 minutes and TTRW measures the time that it takes a patient to run or walk 10 meters. The fixed sequential testing process was applied to the key secondary endpoints in the order listed above.
The primary endpoint and key secondary endpoints were met for the AGAMREE 6 mg/kg/day treatment group. The AGAMREE 2 mg/kg/day treatment group was statistically significant vs. placebo for TTSTAND and 6MWT, but was not statistically significant vs. placebo for TTRW. See Figure 1and Table 2for efficacy results from Week 24.
Figure 1: Least-Squares Mean Change in Time to Stand (TTSTAND) Velocity (rises/sec)
Table 2: Change from Baseline to Week 24 on TTSTAND, 6MWT, and TTRW Compared to Placebo Baseline values are presented with descriptive statistics (mean). Mean changes and differences are model-based least-squares means and mean differences. Positive numbers indicate improvement as compared with the baseline value.
*Primary endpoint
Parameter Placebo AGAMREE
2 mg/kg/dAGAMREE
6 mg/kg/dTTSTAND velocity (rises/sec)
Baseline
Mean change from baseline
Difference from placebo (95% CI)
p-value
0.200
-0.012
N/A
N/A
0.184
0.033
0.045 (0.008, 0.082)
0.017
0.186
0.048
0.060 (0.023, 0.098)
0.002 *6MWT distance (meters)
Baseline
Mean change from baseline
Difference from placebo (95% CI)
p-value
355
-14
N/A
N/A
316
27
40 (13, 68)
0.004
313
29
42 (16, 69)
0.002TTRW velocity (meters/sec)
Baseline
Mean change from baseline
Difference from placebo (95% CI)
p-value
1.735
0.014
N/A
N/A
1.563
0.141
0.127 (-0.026, 0.281)
0.103
1.600
0.258
0.244 (0.093, 0.395)
0.002 -
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
AGAMREE oral suspension is an orange flavored homogeneous white to off-white suspension, containing 40 mg/mL of vamorolone.
AGAMREE is supplied as 100 mL in 125 mL glass bottle packaged with one bottle adapter, two 5 mL oral syringes, and an Instructions for Use: NDC: 69616-264-38.
16.2 Storage and Handling
Store the bottle upright at room temperature between 20°C to 25°C (68°F to 77°F). Excursions permitted between 15°C to 30°C (59°F to 86°F) in the original carton. See USP controlled room temperature.
After opening, store the bottle upright in a refrigerator 2°C to 8°C (36°F to 46°F). Do not freeze.
Discard any unused AGAMREE oral suspension remaining after 3 months of first opening the bottle.
-
17 PATIENT COUNSELING INFORMATION
Advise the patients and/or caregivers to read the FDA-approved patient labeling ( Instructions for Use).
Administration
- Warn patients and/or caregivers to not stop taking AGAMREE abruptly or without first checking with their healthcare providers as there may be a need for gradual dose reductions to decrease the risk of adrenal insufficiency crisis [see Dosage and Administration ( 2.7) and Warnings and Precautions ( 5.1)].
- AGAMREE oral suspension should be taken once daily, preferably with a meal.
- AGAMREE oral suspension must be shaken well for about 30 seconds prior to measuring out each dose with the enclosed oral syringe.
- Discard any unused AGAMREE oral suspension remaining after 3 months of first opening the bottle.
Immunosuppression and Increased Risk of Infection
Tell patients and/or caregivers to inform their healthcare provider if the patient has had recent or ongoing infections or if they have recently received a vaccine. Medical advice should be sought immediately if the patient develops fever or other signs of infection. Patients and/or caregivers should be made aware that some infections can potentially be severe and fatal.
Warn patients who are on corticosteroids to avoid exposure to chickenpox or measles and to alert their healthcare provider immediately if they are exposed [see Warnings and Precautions ( 5.2)] .
Alterations in Cardiovascular/Renal Function
Inform patients and/or caregivers that corticosteroids, including AGAMREE, can cause an increase in blood pressure and water retention. If this occurs, dietary salt restriction and potassium supplementation may be needed [see Warnings and Precautions ( 5.3)].
Behavioral and Mood Disturbances
Advise patients and/or caregivers about the potential for severe behavioral and mood changes with AGAMREE and encourage them to seek medical attention if psychiatric symptoms develop [see Warnings and Precautions ( 5.5)].
Decreases in Bone Mineral Density
Advise patients and/or caregivers about the risk of osteoporosis with prolonged use of AGAMREE, which can predispose the patient to vertebral and long bone fractures [see Warnings and Precautions ( 5.6)].
Ophthalmic Effects
Inform patients and/or caregivers that AGAMREE may cause cataracts or glaucoma and advise monitoring if administered for more than 6 weeks [see Warnings and Precautions ( 5.7)].
Vaccination
Advise patients and/or caregivers to bring immunizations up-to-date according to immunization guidelines prior to starting therapy with AGAMREE. Live-attenuated or live vaccines should be administered at least 4 to 6 weeks prior to starting AGAMREE. Inform patients and/or caregivers that they may receive concurrent vaccinations with use of AGAMREE, except for live-attenuated or live vaccines [see Warnings and Precautions ( 5.8)].
Drug Interactions
Certain medications can cause an interaction with AGAMREE [see Drug Interactions ( 7.1)] . Advise patients and/or caregivers to inform their healthcare provider of all the medicines the patient is taking, including over-the-counter medicines (such as insulin, aspirin or other NSAIDS), dietary supplements, and herbal products. Inform patients and/or caregivers that alternate therapy, dosage adjustment, and/or special test(s) may be needed during the treatment.
Manufactured for:
Catalyst Pharmaceuticals, Inc.
Coral Gables, FL 33134, USAAGAMREE Oral Suspension made in Italy.
AGAMREE ®is a registered trademark of Santhera Pharmaceuticals (Schweiz) AG.
6010 Rev. B.
-
INSTRUCTIONS FOR USE
Instructions for Use
AGAMREE®(ah gam' ree)
(vamorolone)
40 mg/mL oral suspensionRead this Instructions for Use before you start using AGAMREE oral suspension and each time you get a new bottle.
This information does not take the place of talking to your healthcare provider about your medical condition or treatment.Supplies provided in the AGAMREE carton:
- 1 bottle containing 100 mL of AGAMREE, with a child-resistant cap
- 1 bottle adapter
- Two 5 mL oral syringes
- 1 Prescribing information with Instructions for Use

Important information you need to know before taking AGAMREE:
- For oral use only(take by mouth).
- Always use the oral syringes provided with your AGAMREE oral suspension to make sure you measure the right amount.
- Ask your healthcare provider or pharmacist to show you how to measure your prescribed daily dose using the oral syringe.
- Call your pharmacist if your oral syringes are lost or damaged.
- Each oral syringe can be used for 45 days. Call your pharmacist if you need more oral syringes.
- Take AGAMREE exactly as your healthcare provider tells you to take it. Do notstop taking AGAMREE suddenly without first speaking with your healthcare provider.
- AGAMREE oral suspension should be taken 1-time daily with a meal.
- Do notmix the AGAMREE oral suspension with any type of liquids before taking or giving the prescribed daily dose.
- Do notuse AGAMREE 3 months after opening the bottle. Write the date of first opening on your AGAMREE bottle when you first open it.
Storing AGAMREE
- Store the unopened bottle upright at room temperature between 68°F to 77°F (20°C to 25°C) in the original carton. After opening the bottle, store the bottle upright in a refrigerator between 36°F to 46°F (2°C to 8°C).
- Do notfreeze.
- Throw away (discard) any unused AGAMREE oral suspension remaining after 3 months of first opening the bottle.
Keep AGAMREE oral suspension and all medicines out of the reach of children.
For further information call 1-833-422-8259 or go to www.YourCatalystPathways.com.
Manufactured for: Catalyst Pharmaceuticals, Inc., Coral Gables, FL 33134
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Revised: 03/2024
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel – 100 mL Carton Label
NDC: 69616-264-38
100 mL
AGAMREE®
(vamorolone)Oral suspension
Rx only
40 mg/mL
For Oral Administration Only
Catalyst Pharmaceuticals, Inc.

-
PRINCIPAL DISPLAY PANEL
Principal Display Panel – 100 mL Carton Label
NDC: 69616-265-38
100 mL
AGAMREE®
(vamorolone)Oral suspension
Rx only
40 mg/mL
For Oral Administration Only
Date of first Opening:
_______ / ______ / _______

-
INGREDIENTS AND APPEARANCE
AGAMREE
vamorolone kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 69616-264 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69616-264-38 1 in 1 CARTON; Type 1: Convenience Kit of Co-Package 02/01/2024 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE, GLASS 100 mL Part 1 of 1 AGAMREE
vamorolone suspensionProduct Information Item Code (Source) NDC: 69616-265 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VAMOROLONE (UNII: 8XP29XMB43) (VAMOROLONE - UNII:8XP29XMB43) VAMOROLONE 40 mg in 1 mL Inactive Ingredients Ingredient Name Strength SUCRALOSE (UNII: 96K6UQ3ZD4) XANTHAN GUM (UNII: TTV12P4NEE) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM BENZOATE (UNII: OJ245FE5EU) GLYCERIN (UNII: PDC6A3C0OX) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) Product Characteristics Color Score Shape Size Flavor ORANGE (ORANGE) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69616-265-38 100 mL in 1 BOTTLE, GLASS; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA215239 02/01/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA215239 02/01/2024 Labeler - Catalyst Pharmaceuticals, Inc. (053742248)
Trademark Results [AGAMREE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 AGAMREE 90499828 not registered Live/Pending |
Santhera Pharmaceuticals (Schweiz) AG 2021-01-31 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.