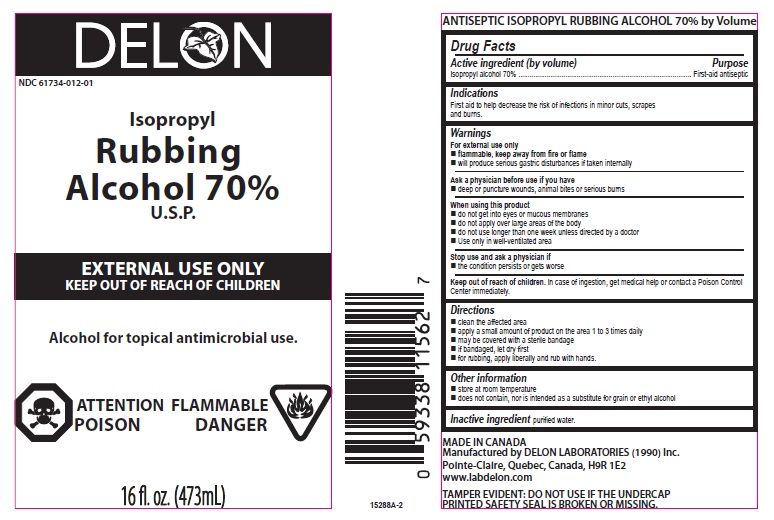
ISOPROPYL RUBBING ALCOHOL 70- isopropyl alcohol solution
Delon Laboratories (1990) Ltd.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Isopropyl Alcohol 70%
Active Ingredient
Isopropyl alcohol 70%
Purpose
First-aid antiseptic
Uses
First aid to help decrease the risk of infections in minor cuts, scrapes and burns.
Warnings
For external use only
-
flammable, keep away from fire or flame
- will produce serious gastric disturbances if taken internally
Ask a physician before use if you have
- deep or puncture wounds, animal bites or serious burns
When using this product
- do not get into eyes or mucous membranes
- do not apply over large areas of the body
- do not use longer than one week unless directed by a doctor
- Use only in well-ventilated area
Stop use and ask physician if
- the condition persists or gets worse
Keep out of reach of children. In case of ingestion, get medical help or contact a Poison Control Center immediately.
Directions
- clean the affected area
- may be covered with a sterile bandage
- if bandaged, let dry first
- for rubbing, apply liberally and rub with hands.
- apply a small amount of product on the area 1 to 3 times daily
Other Information
- store at room temperature
- does not contain, nor is intended as a substitute for grain or ethyl alcohol
Inactive Ingredient
purified water.
ISOPROPYL RUBBING ALCOHOL 70
isopropyl alcohol solution |
| Product Information |
| Product Type | HUMAN OTC DRUG | Item Code (Source) | NDC: 61734-012 |
| Route of Administration | TOPICAL |
|
| Active Ingredient/Active Moiety |
| Ingredient Name | Basis of Strength | Strength |
| ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) | ISOPROPYL ALCOHOL | 70 mL in 100 mL |
|
| Inactive Ingredients |
| Ingredient Name | Strength |
| WATER (UNII: 059QF0KO0R) | |
|
|
| Packaging |
| # | Item Code | Package Description | Marketing Start Date | Marketing End Date |
| 1 | NDC: 61734-012-01 | 473 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product | 10/04/2014 | 09/30/2018 |
| 2 | NDC: 61734-012-02 | 118 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product | 10/04/2014 | 12/04/2014 |
| 3 | NDC: 61734-012-03 | 250 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product | 10/04/2014 | 12/04/2014 |
| 4 | NDC: 61734-012-04 | 1000 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product | 10/04/2014 | 12/04/2014 |
| 5 | NDC: 61734-012-05 | 3780 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product | 10/04/2014 | 12/04/2014 |
| 6 | NDC: 61734-012-06 | 100 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product | 10/04/2014 | 12/04/2014 |
| 7 | NDC: 61734-012-07 | 355 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product | 10/04/2014 | 12/04/2014 |
| 8 | NDC: 61734-012-08 | 946 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product | 10/04/2014 | 12/04/2014 |
| 9 | NDC: 61734-012-09 | 174 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product | 10/04/2014 | 10/31/2019 |
|
|
| Marketing Information |
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph not final | part333A | 05/07/2010 | 10/31/2019 |
|
| Labeler - Delon Laboratories (1990) Ltd.
(248364184)
|
| Establishment |
| Name | Address | ID/FEI | Business Operations |
| Delon Laboratories (1990) Ltd | | 243387722 | label(61734-012) , manufacture(61734-012) , pack(61734-012) |
Delon Laboratories (1990) Ltd.