FELYCIN-CA1 0.4- sirolimus tablet FELYCIN-CA1 1.2- sirolimus tablet FELYCIN-CA1 2.4- sirolimus tablet
felycin-CA1 2.4 by
Drug Labeling and Warnings
felycin-CA1 2.4 by is a Animal medication manufactured, distributed, or labeled by Pegasus Laboratories, Inc., TriviumVet, Concord Biotech Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- felycin®-CA1 (sirolimus delayed-release tablets)
- CAUTION:
-
Description:
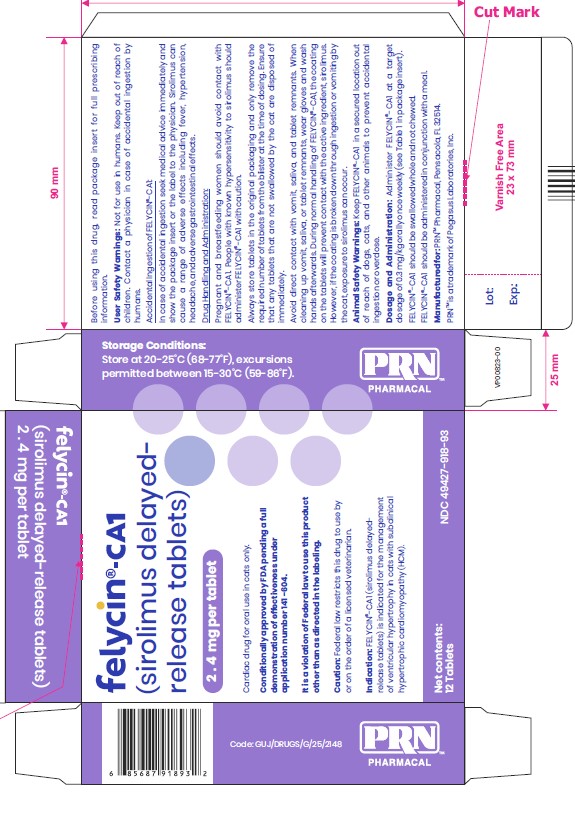
FELYCIN®-CA1 (sirolimus delayed-release tablets) contains the active ingredient sirolimus.
FELYCIN®-CA1 is available in 0.4 mg, 1.2 mg, and 2.4 mg tablet strengths.
FELYCIN®-CA1 are enteric film-coated biconvex tablets, plain on both sides. The 0.4 mg tablet is orange, the 1.2 mg tablet is blue, and the 2.4 mg tablet is white.
-
Indication:
FELYCIN®-CA1 (sirolimus delayed-release tablets) is indicated for the management of ventricular hypertrophy in cats with subclinical hypertrophic cardiomyopathy (HCM).
Subclinical HCM refers to cats with left ventricular (LV) hypertrophy (LV wall thickness of ≥6 mm at end diastole by 2D or M-mode assessment) in the absence of systemic hypertension, other causes of compensatory myocardial hypertrophy, current or historic symptoms of congestive heart failure, arterial thromboembolism, and severe LV outflow tract obstruction.
-
Dosage and Administration:
Administer FELYCIN®-CA1 at a target dosage of 0.3 mg/kg orally once weekly (see Table 1).
FELYCIN®-CA1 should be swallowed whole and not chewed. Do not split or crush tablets.
FELCYIN®-CA1 should be administered in conjunction with a meal.
Table 1: Dosing table (0.3 mg/kg once per week) Number of Tablets Body Weight (kg) 0.4 mg 1.2 mg 2.4 mg 2.5 - 3.2 2 0 0 3.3 - 4.8 0 1 0 4.9 - 6.4 1 1 0 6.5 - 9.6 0 0 1 >9.6 0 1 1 Due to the avaiable tablet strengths, cats weighing less than 2.5 kg cannot be accurately dosed.
-
Contraindications:
Do not use FELYCIN®-CA1 in cats with diabetes mellitus. Discontinue immediately if a cat receiving FELYCIN®-CA1 is diagnosed with diabetes mellitus. The administration of FELYCIN®-CA1 to a cat that developed diabetes mellitus was associated with the development of diabetic ketoacidosis and death. (see Adverse Reactions).
Do not administere FELYCIN®-CA1 in cats with pre-existing liver disease (see Adverse Reactions, Precautions, and Target Animal Safety).
-
Warnings:
User Safety Warnings:
Not for use in humans.
Keep out of reach of children.
Contact a physician in case of accidently ingestion by humans.
Accidental Ingestion of FELYCIN®-CA1:
In case of accidental ingestion seek medical advice immediately and show the package insert or the label to the physician.
Sirolimus can cause a range of adverse effects including fever, hypertension, headache, and adverse gastrointestinal effects.
Drug Handling and Administration:
Pregnant and breastfeeding women should avoid contact with FELYCIN®-CA1.
People with known hypersenstivity to sirolimus should administer FELYCIN®-CA1 with caution.
Always store tablets in the original packaging and only remove the required number of tablets from the blister at the time of dosing.
Ensure that any tablets that are not swallowed by the cat are disposed of immediately.
Avoid direct contact with vomit, saliva, or tablet remnants. When cleaning up vomit, saliva, or tablet remnants, wear gloves and wash hands afterwards.
During normal handling of FELYCN®-CA1, the coating on the tablets will prevent contact with the active ingredient, sirolimus. However, if the coating is broken down through ingestion or vomiting by the cat, exposure to sirolimus can occur.
To obtain a copy of the Safety Data Sheet (SDS), contact PRN Pharmacal at 1-800-874-9764.
Animal Safety Warnings:
Sirolimus is a known substrate for cytochrome P-450 3A4 (CYP 3A4) and P-glycoprotein (P-gp) in humans. Administration of FELYCIN®-CA1 with drugs that inhibit CYP 3A4 or P-gp, such as calcium channel blockers, amiodarone, azoles (e.g. ketoconazole), or cyclosporine, may increase risk for toxicity. Use caution when administering FELYCIN®-CA1 in cats with the MDR1 mutation or when administering concomitantly with another P-gp substrate (e.g. eprinomectin and emodepside).
Treatment with FELYCIN®-CA1 could impact the cat's ability to mount an adequate immune response to vaccinations.
Concurrent administration of FELYCIN®-CA1 did not impact the cat's ability to mount an adequate immune reponse to a killed rabies vaccine (see Clinical Pharmacology and Target Animal Safety). The impact of concurrent administration of FELYCIN®-CA1 on vaccination for FHV-1, FCV, FPV, and FeLV has not been evaluated.
Keep FELYCIN®-CA1 in a secure location out of reach of dogs, cats and other animals to prevent accidental ingestion or overdose.
-
Precautions:
For use only in otherwise healthy cats with subclinical HCM in the absence of other causes of compensatory myocardial hypertrophy (e.g., systemic hypertension), current or historic symptoms of congestive heart failure, arterial thromboembolism, and severe LV outflow tract obstruction.
A diagnosis of subclinical HCM should be made by means of a comprehensive physical examination including blood pressure measurement to rule out systemic hypertension, and cardiac examination which should include echocardiography to confirm the presence of LV hypertrophy and radiography to rule out congestive heart failure.
Echocardiographic examinnation is recommended in all cases to diagnose subclinical HCM. A diagnosis of subclinical HCM is based on an end-diastolic left ventricular wall thickness of ≥6 mm measured by 2D or M-mode assessment.
Sirolimus undergoes extensive hepatic metabolism in humans. Prior to initiation of treatment with FELYCIN®-CA1, a comprehensive physical examination and screening bloodwork including a serum biochemical profile should be conducted to rule out pre-existing liver dysfunction.
Treatment with FELYCIN® -CA1 has been associated with the elevation of the transaminase enzymes, which include alanine aminotransferase (ALT) and aspartate aminotransferase (AST).
Bloodwork should be repeated 1 to 2 months following initiation of treatment, and every 6 to 12 months thereafter. If mild transaminase elevations are observed (up to 2X the upper limit of normal (ULN)), bloodwork should be repeated in
2 months. If these values remain elevated, discontinue treatment with FELYCIN®-CA1.Discontinue treatment with FELYCIN®-CA1 if transaminase values exceed 2X the upper limit of normal, if other liver enzymes besides ALT or AST are elevated, or if clinical signs of liver dysfunction are noted.
Available information does not indicate that FELYCIN®-CA1 is immunosuppressive at the doses administered. The use of FELYCIN®-CA1 in cats with chronic viral diseases like feline viral rhinotracheitis has not been evaluated.
The safety and effectiveness of FELYCIN®-CA1 has not been evaluated in cats with other cardiomyopathy phenotypes.
The safety and effectiveness of FELYCIN®-CA1 has not been evaluated in cats receiving beta blockers or corticosteroids.
The safety and effectiveness of FELYCIN®-CA1 has not been evaluated in cats with chronic kidney disease, hyperthyroidism, or other significant systemic disease.
The effectiveness of FELYCIN®-CA1 has not been evaluated in sexually intact cats. Therefore, FELYCIN®-CA1 should not be used in animals intended for breeding.
-
Adverse Reactions:
In a well-controlled pilot field study, 43 cats with subclinical HCM were administered either the label dose of FELYCIN®-CA1 (0.3 mg/kg once
weekly; n=15), twice the label dose (0.6 mg/kg once weekly; n=15), or a placebo control tablet (n=13).Cats were followed for 180 days or until removal from the study (see Reasonable Expectation of Effectiveness).Cardiac: The most frequently observed adverse reactions in cats treated with FELYCIN®-CA1 were cardiovascular in nature, relating to the
progression of HCM, and included arrhythmia, congestive heart failure, syncope, and pericardial effusion.Three of the cats receiving twice the label dose of FELYCIN®-CA1 (0.6 mg/kg) progressed to congestive heart failure or sudden death. Two of
these cats had severe pre-existing structural disease. The third cat did not have severe structural disease at enrollment but had
markedly elevated serum N-terminal pro-brain natriuretic peptide (NTproBNP) at enrollment (1344 pmol/L, normal <100 pmol/L), which can
indicate an increased risk of disease progression. The relationship to treatment with FELYCIN®-CA1 is unknown due to the small sample size of this study and the variable disease progression of HCM.Non-Cardiac: Other adverse reactions observed in cats treated with FELYCIN®-CA1 were lethargy, vomiting, diarrhea, and inappetence.
Diabetes Mellitus: One cat receiving the label dose (0.3 mg/kg) of FELYCIN®-CA1 developed diabetes mellitus during the study, manifesting as
hypercholesterolemia, hyperglycemia, and glucosuria with prior evidence of urinary tract infection at scheduled visits. Treatment for
diabetes was not initiated and the cat continued on the study. Subsequently, the cat presented in diabetic ketoacidosis, and despite intensive
medical management, the cat died of acute cardiac arrest.Pre-Existing Liver Disease: In a separate pilot field study conducted in cats with chronic kidney disease (CKD), one cat was enrolled with a history
of elevated alkaline phosphatase (ALP). After treatment with the label dose (0.3 mg/kg) of FELYCIN®-CA1, this cat experienced a progressive
decline in appetite, elevation of liver enzymes, including ALP, ALT, and AST, and icterus, and was euthanized approximately 4 months after exiting the study. - Contact Information:
-
Clinical Pharmacology:
Mode of Action:
Sirolimus is an immunosuppressant that targets and inhibits the mammalian target of rapamycin C1 (mTORC1) protein complex, a central regulator of cell growth and nutrient response. Studies in rodent models suggest mTOR inhibition by sirolimus attenuates cardiac hypertrophy by promoting autophagy, attenuating oxidative stress and blocking pro-inflammatory responses, thereby resulting in an improvement in cardiac function in rodents.Pharmacokinetics:
In a laboratory safety study in healthy adult cats after repeat oral dosing of FELYCIN®-CA1 once per week for 24 weeks (See Target Animal Safety), mean dose normalized maximum plasma concentration (Cmax) values decreased with an increasing dose suggesting that absorption of sirolimus may be saturated at higher dosing levels in cats. The comparison between the area under the curve from dosing extrapolated to infinity (AUCinf) at Day 0 and area under the curve from the time of dosing to the last quantifiable concentration (AUClast) at Day 147 suggests the pharmacokinetics are non-linear after multiple dosing.At 0.38 mg/kg, accumulation was observed between Days 0 and 147 with geometric mean accumulation ratios for the Cmax and area under the curve AUClast of 1.33 and 1.62, respectively.
Table 2 Arithmetic mean (±standard deviation) of sirolimus pharmacokinetic parameters following the first administration of FELYCIN®-CA1 (maximum proposed label dose 0.38 mg per kg body weight) in male and female cats in a laboratory study. Parameter Estimate AUClast(h*ng/mL) 288 ± 198 Cmax(ng/mL) 22.0 ± 15.8 t1/2(h) 71.8 ± 42.0 Tmax(h)* 1.50 (1.00-12.0) AUClast = area under the curve from dosing to 168 hours
Cmax = maximum plasma concentrationt1/2 = half-life
Tmax= time to maximum plasma concentration*Median (range)
-
Reasonable Expectation of Effectiveness:
A reasonable expectation of effectiveness may be demonstrated based on evidence such as, but not limited to, pilot data in the target species or studies from published literature.
FELYCIN®-CA1 is conditionally approved pending a full demonstration of effectiveness.Additional information for Conditional Approvals can be found at www.fda.gov/animalca.
A reasonable expectation of effectiveness for FELYCIN®-CA1 is based on published scientific literature and results from a pilot field study conducted at two US referral cardiology centers.
Published literature, including studies in a mouse model of concentric left ventricular (LV) hypertrophy and in human patients following cardiac transplantation, demonstrated that sirolimus decreased LV hypertrophy and improved diastolic function.
Pilot Field Study:
A well-controlled pilot field study enrolled a total of 43 cats of various breeds. The cats received either FELYCIN®-CA1 at the label dose of 0.3 mg/kg once weekly (n=15), FELYCIN®-CA1 at 0.6 mg/kg once weekly (n=15), or placebo control tablets once weekly (n=13).Cats ranged between 1 and 12 years of age and weighed between 3.3 and 14 kg at enrollment; 37 of the 43 cats were male. Cats were confirmed to have evidence of subclinical HCM prior to enrollment based on echocardiographic findings of LV hypertrophy (LV wall thickness of ≥6 mm at end diastole by 2D or M-mode assessment), with no evidence of congestive heart failure (CHF), arterial thromboembolism, or arrhythmias requiring specific anti-arrhythmic therapy. Cats were ineligible if they were found to have evidence of cardiogenic pulmonary edema, severe LV outflow tract obstruction (LV outflow tract gradient ≤50 mmHg), clinically significant tachyarrhythmias, cardiac disease other than HCM, systemic hypertension, significant systemic di sease, or were recei vi ng l ong- term corticosteroid treatment. Concomitant use of oral clopidogrel and/or angiotensin-converting enzyme (ACE) inhibitors was permitted if administered for at least 2 weeks prior to study enrollment. No new cardiac medications were permitted during the study.
Exploratory analyses were conducted evaluating measures of LV hypertrophy and left atrial dilation in addition to comparing the relationship between disease progression/response and baseline patient characteristics. Effectiveness was based on changes in maximum wall thickness (MWT) of the LV.
Of the 43 cats enrolled in the study, 36 cats were still enrolled at the final evaluation on Day 180. Six cats (5 high dose and 1 control) were excluded due to the progression of heart disease, death, or owner removal.
Echocardiographic values were comparable between the three study groups at baseline. Following 180 days of treatment, differences in LV MWT were evident. Cats treated with 0.3 mg/kg (label dose) of FELYCIN®-CA1 had a lower mean MWT and the difference between the 0.3 mg/kg FELYCIN®-CA1 group and the control group was statistically significant across Day 60 and Day 180. No statistically significant treatment effects were detected for other echocardiographic values.
Within the 36 evaluable cases at Day 180, MWT decreased by a mean value of 0.17 mm in the 0.3 mg/kg (label dose) group (n=14). In contrast, MWT increased by a mean of 0.94 mm in the placebo group (n=12), and by 0.50 mm in the 0.6 mg/kg group (n=10).
-
Target Animal Safety:
Margin of Safety Study:
A 24-week laboratory margin of safety study was conducted in 32 healthy laboratory cats, aged 10 to 11 months at enrollment. Cats were randomized into 4 groups of 8 cats with 4 male and 4 female cats in each group. FELYCIN®-CA1 was administered at a dose of 0, 0.38, 1.13, and
1.88 mg/kg (0X, 1.3X, 3.8X, and 6.3X the label dose of 0.3 mg/kg) for 24 weeks. Cats were dosed in a fed state and cats in the control group were untreated. No clinically significant effects on physical examination, food consumption, bodyweight, or postmortem examination parameters were identified.Of the 24 cats that received FELYCIN®-CA1, 15 cats experienced at least one transaminase value elevation (i.e., AST and ALT) during the course of the study. These cats were in all 3 FELYCIN®-CA1 dosing groups and a dose-response was not present. Transaminase elevations were not observed in the control cats. Some of the elevations were transient and/or mild (i.e., less than 2X the upper limit of the reference range). Four male littermates were found to have the most severe elevations, with maximum AST values of 110 to 515 U/L (reference range 16 to 34 U/L) and maximum ALT values of 255 to 4552 U/L (reference range 41 to 160 U/L). Transaminase elevations were recorded at the first bloodwork collection time point (Day 26/27) after the treatment initiation in 3 of the 4 littermates, and elevations were present throughout the 24-week dosing period. All affected cats remained clinically normal and postmortem examination revealed no signs of liver pathology.
Pilot Safety Study:
In a pilot laboratory study, 32 cats (11 males and 21 females, aged 2 to 5 years) were allocated to 4 groups of 8 cats. FELYCIN®-CA1 was administered 3 times per week at doses of 0, 0.15, 0.45, or 0.75 mg/kg (0X, 1.5X, 4.5X, or 7.5X the label dose) for 4 weeks, followed by a 4-week recovery period. The clinical observations, physical examinations, and body weight evaluations did not reveal findings of clinical or toxicological significance during the study. Mild transaminase elevations were observed in 8 of the 24 cats receiving FELYCIN®-CA1. By Day 55, all ALT values were within the reference range and AST values were either within the reference range or showing a downward trend after the discontinuation of treatment with FELYCIN®-CA1.Vaccine Response Study:
In a laboratory study, 20 healthy, vaccine-naïve cats (4 per sex in the control group and 6 per sex in the treated group), approximately 4 months of age at study initiation were administered FELYCIN®-CA1 at 0 and 0.9 mg/kg (0X and 3X the label dose) once a week for 56 days (Days 0, 7, 14, 21, 28, 35, 42, 49, and 56). Cats were dosed in a fed state and the control cats were sham dosed. A commercially available killed rabies vaccine was administered to all cats on Day 29. All cats (control and treated) in the study demonstrated an adequate immune (serologic) response to the killed rabies virus vaccine on Day 57. The clinical observations, physical examinations, and clinical pathology assessments revealed no clinically significant abnormal findings. - How supplied:
- Storage Conditions:
- Manufactured For:
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FELYCIN-CA1 0.4
sirolimus tabletProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 49427-916 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SIROLIMUS (UNII: W36ZG6FT64) (SIROLIMUS - UNII:W36ZG6FT64) SIROLIMUS 0.4 mg Product Characteristics Color orange Score no score Shape ROUND Size 5mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 49427-916-93 12 in 1 CARTON 1 1 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date conditional NADA NADA141604 06/21/2025 FELYCIN-CA1 1.2
sirolimus tabletProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 49427-917 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SIROLIMUS (UNII: W36ZG6FT64) (SIROLIMUS - UNII:W36ZG6FT64) SIROLIMUS 1.2 Product Characteristics Color blue Score no score Shape ROUND Size 6mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 49427-917-93 12 in 1 CARTON 1 1 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date conditional NADA NADA141604 06/21/2025 FELYCIN-CA1 2.4
sirolimus tabletProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 49427-918 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SIROLIMUS (UNII: W36ZG6FT64) (SIROLIMUS - UNII:W36ZG6FT64) SIROLIMUS 2.4 mg Product Characteristics Color white Score no score Shape ROUND Size 6mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 49427-918-93 12 in 1 CARTON 1 1 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date conditional NADA NADA141604 06/21/2025 Labeler - Pegasus Laboratories, Inc. (108454760) Registrant - TriviumVet (985627951) Establishment Name Address ID/FEI Business Operations Concord Biotech Limited 650996940 analysis, label, manufacture, pack Establishment Name Address ID/FEI Business Operations Concord Biotech Limited 916708337 api manufacture
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.