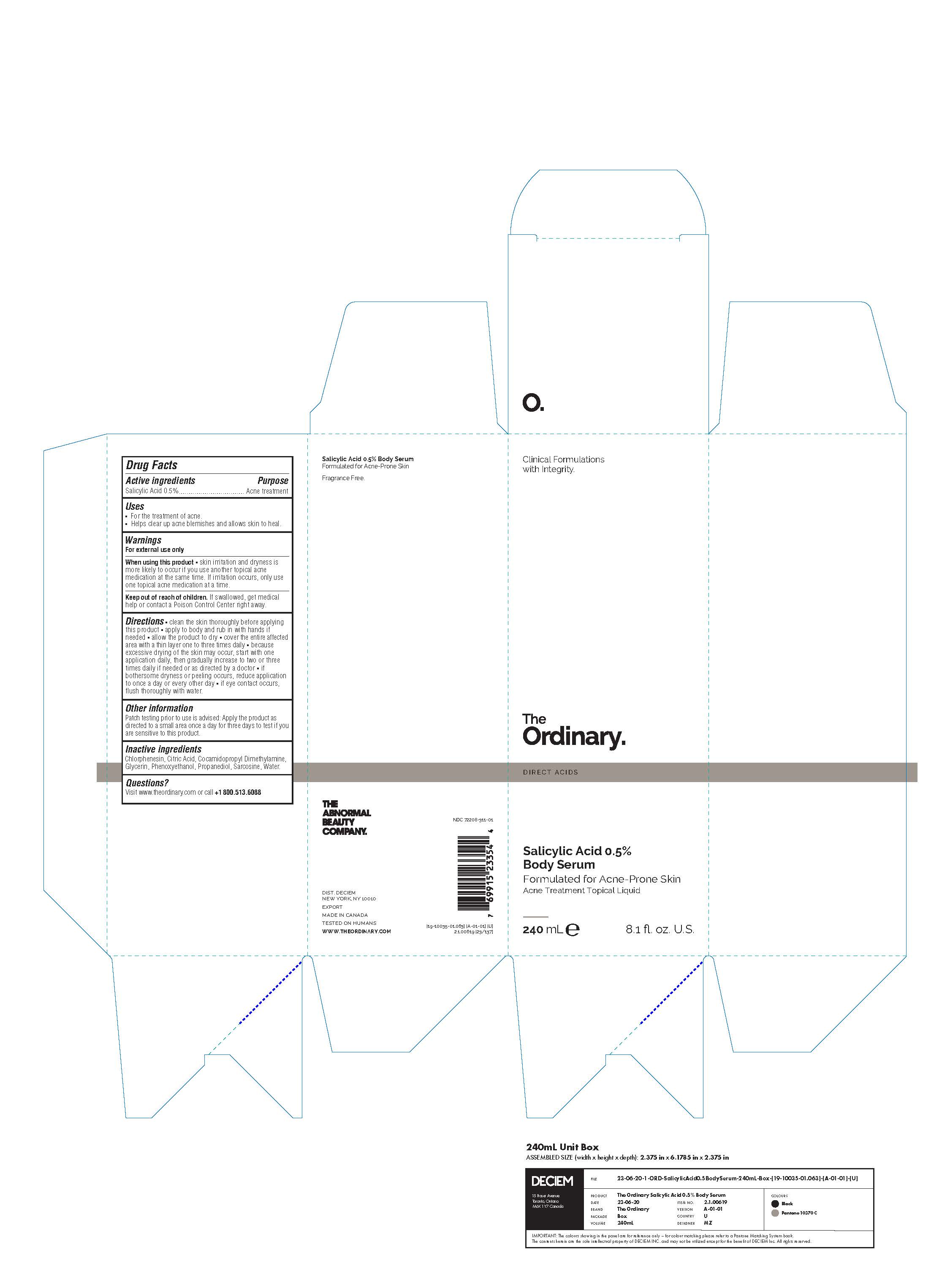
The Ordinary Salicylic Acid 0.5%
The Ordinary Salicylic Acid 0.5% by
Drug Labeling and Warnings
The Ordinary Salicylic Acid 0.5% by is a Otc medication manufactured, distributed, or labeled by Deciem Inc, Crystal Claire Cosmetics Inc., DECIEM Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
THE ORDINARY SALICYLIC ACID 0.5%- salicylic acid lotionÂ
Deciem Inc
----------
The Ordinary Salicylic Acid 0.5%
Warnings
For external use only
When using this product  skin irritation and dryness is
more likely to occur if you use another topical acne
medication at the same time. If irritation occurs, only use
one topical acne medication at a time.
Keep out of reach of children. If swallowed, get medical
help or contact a Poison Control Center right away.
Directions  clean the skin thoroughly before applying
this product  apply to body and rub in with hands if
needed  allow the product to dry  cover the entire affected
area with a thin layer one to three times daily  because
excessive drying of the skin may occur, start with one
application daily, then gradually increase to two or three
times daily if needed or as directed by a doctor  if
bothersome dryness or peeling occurs, reduce application
to once a day or every other day  if eye contact occurs,
flush thoroughly with water.
| THE ORDINARY SALICYLIC ACID 0.5%Â
salicylic acid lotion |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler -Â Deciem Inc (203133665) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Crystal Claire Cosmetics Inc. | 205493484 | analysis(72208-311) , manufacture(72208-311) , label(72208-311) , pack(72208-311) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| DECIEM Inc | 243327654 | label(72208-311) , manufacture(72208-311) , pack(72208-311) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.