ANTIGEN COMPONENT by Sanofi Pasteur Inc. ANTIGEN COMPONENT
ANTIGEN COMPONENT by
Drug Labeling and Warnings
ANTIGEN COMPONENT by is a Other medication manufactured, distributed, or labeled by Sanofi Pasteur Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ANTIGEN COMPONENT- cov-2 pres dtm antigen injection, emulsion
Sanofi Pasteur Inc.
----------
ANTIGEN COMPONENT
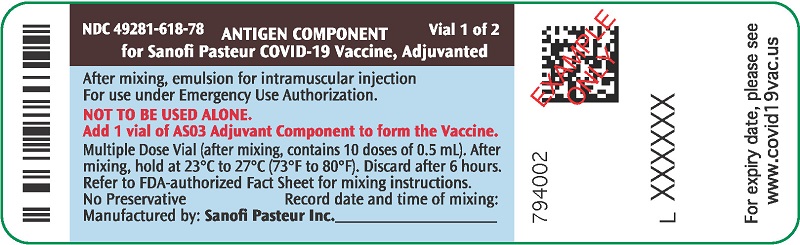
PRINCIPAL DISPLAY PANEL - 10 Dose Vial Label
NDC: 49281-618-78
Vial 1 of 2
ANTIGEN COMPONENT
for Sanofi Pasteur COVID-19 Vaccine, Adjuvanted
After mixing, emulsion for intramuscular injection
For use under Emergency Use Authorization.
NOT TO BE USED ALONE.
Add 1 vial of AS03 Adjuvant Component to form the Vaccine.
Multiple Dose Vial (after mixing, contains 10 doses of 0.5 mL). After
mixing, hold at 23°C to 27°C (73°F to 80°F). Discard after 6 hours.
Refer to FDA-authorized Fact Sheet for mixing instructions.
No Preservative
Record date and time of mixing:
Manufactured by: Sanofi Pasteur Inc.
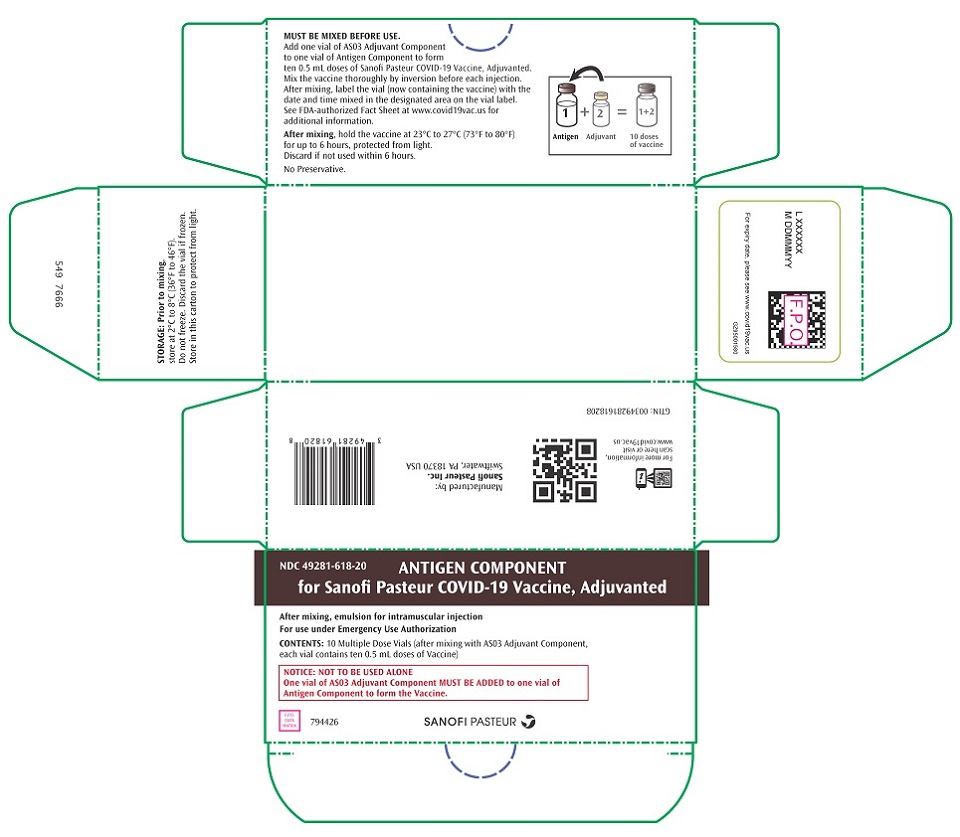
PRINCIPAL DISPLAY PANEL - 10 Vial Carton
NDC: 49281-618-20
ANTIGEN COMPONENT
for Sanofi Pasteur COVID-19 Vaccine, Adjuvanted
After mixing, emulsion for intramuscular injection
For use under Emergency Use Authorization
CONTENTS: 10 Multiple Dose Vials (after mixing with AS03 Adjuvant Component,
each vial contains ten 0.5 mL doses of Vaccine)
NOTICE: NOT TO BE USED ALONE
One vial of AS03 Adjuvant Component MUST BE ADDED to one vial of
Antigen Component to form the Vaccine.
794426
SANOFI PASTEUR
| ANTIGEN COMPONENT
cov-2 pres dtm antigen injection, emulsion |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Sanofi Pasteur Inc. (086723285) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sanofi Pasteur Inc. | 086723285 | MANUFACTURE(49281-618) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.