ONMEL- itraconazole tablet
Onmel by
Drug Labeling and Warnings
Onmel by is a Prescription medication manufactured, distributed, or labeled by Merz Pharmaceuticals, LLC, Sebela Pharmaceuticals Inc., Sanico N.V.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ONMEL safely and effectively. See full prescribing information for ONMEL.
ONMEL (itraconazole)
Initial U.S. Approval: 1992WARNING: CONGESTIVE HEART FAILURE, CARDIAC EFFECTS AND DRUG INTERACTIONS
See full prescribing information for complete boxed warning.
- Do not administer for the treatment of onychomycosis in patients with evidence of ventricular dysfunction such as congestive heart failure (CHF) or a history of CHF ( 4).
- If signs or symptoms of congestive heart failure occur during administration, discontinue administration. ( 4)
- Negative inotropic effects were seen when itraconazole was administered intravenously to dogs and healthy human volunteers. ( 5.3)
- Drug Interactions: Co-administration of certain drugs is contraindicated. See complete boxed warning. ( 7)
- May increase plasma concentrations of drugs metabolized by the cytochrome P450 3A4 isoenzyme system (CYP3A4) pathway. ( 7)
- Serious cardiovascular events, including QT prolongation, torsades de pointes, ventricular tachycardia, cardiac arrest, and/or sudden death have occurred in patients using certain drugs. See complete boxed warning. ( 5.2)
INDICATIONS AND USAGE
- ONMEL, an azole antifungal, is indicated for the treatment of onychomycosis of the toenail caused by Trichophyton rubrum or T. mentagrophytes. ( 1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
- Tablets: 200 mg ( 3)
CONTRAINDICATIONS
- Do not administer for the treatment of onychomycosis in patients with evidence of ventricular dysfunction such as congestive heart failure (CHF) or a history of CHF. ( 4)
- Do not administer for the treatment of onychomycosis to pregnant patients or to women contemplating pregnancy. ( 4, 8.1)
- Co-administration of cisapride, dofetilide, ergot alkaloids such as dihydroergotamine, ergotamine, ergometrine (ergonovine), and methylergometrine (methylergonovine); felodipine, levacetylmethadol (levomethadyl), lovastatin, methadone, oral midazolam, nisoldipine, pimozide, quinidine, simvastatin, and triazolam with ONMEL is contraindicated. ( 4)
- Anaphylaxis and hypersensitivity have been reported with use of itraconazole. ONMEL is contraindicated in patients who have shown hypersensitivity to itraconazole products. ( 4)
WARNINGS AND PRECAUTIONS
- Cases of CHF, peripheral edema, and pulmonary edema have been reported with itraconazole administration among patients being treated for onychomycosis and/or systemic fungal infections. ( 5.5)
- Cardiac Dysrhythmias: ( 5.2)
- Cardiac Disease : ( 5.3)
- Hepatic Effects: ( 5.4)
- Calcium Channel Blockers : ( 5.5)
- Neuropathy: ( 5.6)
- Hearing Loss: ( 5.7)
ADVERSE REACTIONS
- Most common adverse reactions observed in the treatment phase of the onychomycosis clinical trial (>1%) are upper respiratory tract infections, increased hepatic enzymes, hypoacusis, headache, abdominal pain, diarrhea, nausea, fatigue, arrhythmia, cough, sore throat and back pain. ( 6.1)
- Itraconazole has been associated with rare cases of serious hepatotoxicity, including liver failure and death. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Merz Pharmaceuticals, LLC at 1-877-743-8454 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Concomitant administration of ONMEL Tablets with certain drugs metabolized by the cytochrome P450 3A4 isoenzyme system (CYP3A4) or transported by P-glycoprotein may result in increased plasma concentrations of those drugs, leading to potentially serious and/or life-threatening adverse events. ( 7.1)
- Drug Interactions with the following drugs or classes of drugs may occur: Antiarrhythmics, Anticonvulsants, Anti-HIV Agents, Antimycobacterials, Antineoplastics, Antipsychotics, Benzodiazepines, Calcium Channel Blockers, Gastric Acid Suppressors/Neutralizers, Gastrointestinal Motility Agents, HMG CoA-Reductase Inhibitors, Macrolide Antibiotics, Oral Hypoglycemic Agents, Polyenes, Opiate Analgesics. Not all drug interactions are included in Highlights. See Full Prescribing Information for complete listing. ( 7)
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 11/2012
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: CONGESTIVE HEART FAILURE, CARDIAC EFFECTS, AND DRUG INTERACTIONS
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Congestive Heart Failure, Peripheral Edema, and Pulmonary Edema
5.2 Cardiac Dysrhythmias
5.3 Cardiac Disease
5.4 Hepatic Effects
5.5 Calcium Channel Blockers
5.6 Neuropathy
5.7 Hearing Loss
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Post Marketing Experience
7 DRUG INTERACTIONS
7.1 Effects of ONMEL on Other Drugs
7.2 Effects of Other Drugs on ONMEL
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
17.1 Information for Patients
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: CONGESTIVE HEART FAILURE, CARDIAC EFFECTS, AND DRUG INTERACTIONS
Do not administer ONMEL for the treatment of onychomycosis in patients with evidence of ventricular dysfunction such as congestive heart failure (CHF) or a history of CHF. When itraconazole was administered intravenously to dogs and healthy human volunteers, negative inotropic effects were seen. If signs or symptoms of congestive heart failure occur during administration of ONMEL, discontinue administration. [See Contraindications (4), Warnings and Precautions (5), Drug Interactions (7), and Clinical Pharmacology (12)]
Drug Interactions: Co-administration of cisapride, pimozide, quinidine, dofetilide, levacetylmethadol (levomethadyl), felodipine, oral midazolam, nisoldipine, triazolam, lovastatin, simvastatin, ergot alkaloids such as dihydroergotamine, ergometrine (ergonovine), ergotamine and methylergometrine (methylergonovine) or methadone with ONMEL is contraindicated. ONMEL, a potent cytochrome P450 3A4 isoenzyme system (CYP3A4) inhibitor, may increase plasma concentrations of drugs metabolized by this pathway. Serious cardiovascular events, including QT prolongation, torsades de pointes, ventricular tachycardia, cardiac arrest, and/or sudden death have occurred in patients using cisapride, pimozide, levacetylmethadol (levomethadyl), methadone or quinidine concomitantly with itraconazole and/or other CYP3A4 inhibitors. [See Contraindications (4), Warnings and Precautions (5), and Drug Interactions (7)]
-
1 INDICATIONS AND USAGE
ONMEL is indicated for the treatment of onychomycosis of the toenail due to Trichophyton rubrum or T. mentagrophytes in non-immunocompromised patients. Prior to initiating treatment, appropriate nail specimens for laboratory testing (KOH preparation, fungal culture, or nail biopsy) should be obtained to confirm the diagnosis of onychomycosis. [ See Contraindications (4), Warnings and Precautions (5), Drug Interactions (7), and Clinical Pharmacology (12).]
-
2 DOSAGE AND ADMINISTRATION
ONMEL should be taken with a full meal at the same time each day. The recommended dose is 200 mg (one tablet) once daily for 12 consecutive weeks.
Use in Patients with Renal Impairment:
Limited data are available on the use of oral itraconazole in patients with renal impairment. Caution should be exercised when ONMEL is administered to patients with renal impairment. [See Clinical Pharmacology (12) and Warnings and Precautions (5).]
Use in Patients with Hepatic Impairment:
Limited data are available on the use of oral itraconazole in patients with hepatic impairment. Caution should be exercised when ONMEL is administered to patients with hepatic impairment. [See Clinical Pharmacology (12) and Warnings and Precautions (5).]
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Congestive Heart Failure: Do not administer ONMEL for the treatment of onychomycosis in patients with evidence of ventricular dysfunction such as congestive heart failure (CHF) or a history of CHF. [See Warnings and Precautions (5), Drug Interactions (7), and Clinical Pharmacology (12).]
Drug Interactions: Concomitant administration of ONMEL and certain drugs that are metabolized by the cytochrome P450 3A4 isoenzyme system (CYP3A4) or where gastrointestinal absorption is regulated by P-gp may result in increased plasma concentrations of those drugs, leading to potentially serious and/or life-threatening adverse events.
Co-administration of cisapride, dofetilide, ergot alkaloids such as dihydroergotamine, ergotamine, ergometrine (ergonovine), and methylergometrine (methylergonovine), felodipine, levacetylmethadol (levomethadyl), lovastatin, methadone, oral midazolam, nisoldipine, pimozide, quinidine, simvastatin, and triazolam with ONMEL is contraindicated.
Do not administer ONMEL for the treatment of onychomycosis to pregnant patients or to women contemplating pregnancy.
Anaphylaxis and hypersensitivity have been reported with use of itraconazole. ONMEL is contraindicated for patients who have shown hypersensitivity to itraconazole products.
-
5 WARNINGS AND PRECAUTIONS
5.1 Congestive Heart Failure, Peripheral Edema, and Pulmonary Edema
Cases of CHF, peripheral edema, and pulmonary edema have been reported with itraconazole administration among patients being treated for onychomycosis and/or systemic fungal infections. [ See Contraindications (4), Warnings and Precautions (5), and Clinical Pharmacology (12).]
5.2 Cardiac Dysrhythmias
Life-threatening cardiac dysrhythmias and/or sudden death have occurred in patients using cisapride, pimozide, levacetylmethadol (levomethadyl), methadone, or quinidine concomitantly with itraconazole and/or other CYP3A4 inhibitors. Concomitant administration of these drugs with ONMEL is contraindicated. [See Boxed Warning, Contraindications (4), Warnings and Precautions (5), and Drug Interactions (7).]
5.3 Cardiac Disease
ONMEL should not be administered in patients with evidence of ventricular dysfunction such as congestive heart failure (CHF) or a history of CHF.
Itraconazole has been shown to have a negative inotropic effect. When itraconazole was administered intravenously to anesthetized dogs, a dose-related negative inotropic effect was documented. In a healthy volunteer study of itraconazole injection, transient, asymptomatic decreases in left ventricular ejection fraction were observed using gated SPECT imaging; these resolved before the next infusion, 12 hours later.
For patients with risk factors for congestive heart failure, physicians should carefully review the risks and benefits of ONMEL therapy. These risk factors include cardiac disease such as ischemic and valvular disease; significant pulmonary disease such as chronic obstructive pulmonary disease; and renal failure and other edematous disorders. Such patients should be informed of the signs and symptoms of CHF, should be treated with caution, and should be monitored for signs and symptoms of CHF during treatment. If signs or symptoms of CHF appear during administration of ONMEL, discontinue administration.
5.4 Hepatic Effects
Itraconazole has been associated with rare cases of serious hepatotoxicity, including liver failure and death. Some of these cases had neither pre-existing liver disease nor a serious underlying medical condition, and some of these cases developed within the first week of treatment. If clinical signs or symptoms develop that are consistent with hepatotoxicity, treatment should be discontinued immediately and liver function testing performed.
In patients with elevated or abnormal liver enzymes or active liver disease, or who have experienced liver toxicity with other drugs, treatment with itraconazole is not recommended. Liver function monitoring should be done in patients with pre-existing hepatic function abnormalities or those who have experienced liver toxicity with other medications and should be considered in all patients receiving ONMEL.
5.5 Calcium Channel Blockers
Calcium channel blockers can have negative inotropic effects which may be additive to those of itraconazole. In addition, itraconazole can inhibit the metabolism of calcium channel blockers. Therefore, caution should be used when co-administering itraconazole and calcium channel blockers due to an increased risk of CHF. Concomitant administration of ONMEL and nisoldipine is contraindicated.
5.6 Neuropathy
If neuropathy occurs that may be attributable to ONMEL, the treatment should be discontinued.
5.7 Hearing Loss
Transient or permanent hearing loss has been reported in patients receiving treatment with itraconazole. Several of these reports included concurrent administration of quinidine which is contraindicated. [See Boxed Warning, Warnings and Precautions (5), and Drug Interactions (7).] The hearing loss usually resolves when treatment is stopped, but can persist in some patients.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, the adverse reaction rate observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Patients in the trial for toenail onychomycosis were treated with a dosing regimen of 200 mg once daily for 12 consecutive weeks.
The most commonly reported adverse reaction leading to discontinuation of ONMEL was increased hepatic enzyme (6 subjects, 1.0%), followed by dizziness (3 subjects, 0.5%). No other adverse reaction leading to discontinuation occurred in more than one subject.
The table below lists all adverse reactions reported by at least 1% of patients who received ONMEL during 12 weeks of treatment:
Table 1: Adverse Reactions Occurring at Frequencies ≥ 1% in the Onychomycosis Clinical Trial Incidence (%) Incidence (%) BODY SYSTEM/ADVERSE REACTION ONMEL Placebo tablet (N = 582) (N = 191) INFECTIONS AND INFESTATIONS Upper respiratory tract infections 6.0% 7.3% Bacteriuria 1.4% 1.6% Urinary tract infection 1.0% 0.5% I NVESTIGATIONS Hepatic enzymes increased 2.9% 0.0% Electrocardiogram abnormal 1.4% 1.6% EAR AND LABYRINTH DISORDERS Hypoacusis 3.3% 3.1% NERVOUS SYSTEM DISORDERS Headache 2.2% 1.6% Dizziness 1.2% 0.0% GASTROINTESTINAL DISORDERS Abdominal pain or discomfort 1.7% 2.6% Diarrhea 1.7% 3.1% Nausea 1.7% 1.6% GENERAL DISORDERS OF ADMINISTRATION SITE CONDITIONS Fatigue 1.5% 2.6% CARDIAC DISORDERS Sinus Bradycardia 1.0% 0.0% RESPIRATORY, THORACIC AND MEDIASTINAL DISORDERS Cough 1.2% 0.0% Pharyngolaryngeal pain 1.0% 0.5% MUSCULOSKELETAL AND CONNECTIVE TISSUE DISORDERS Back pain 1.2% 2.1% 6.2 Post Marketing Experience
The following adverse reactions have been identified during post-approval use of itraconazole (all formulations) and are listed in Table 2 below. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establishing a causal relationship to drug exposure.
Table 2: Postmarketing Reports of Adverse Reactions for Itraconazole Blood and lymphatic system disorders: Leukopenia, neutropenia, thrombocytopenia Immune system disorders: Anaphylaxis; anaphylactic, anaphylactoid and allergic reactions; serum sickness; angioneurotic edema Metabolism and nutritional disorders: Hypertriglyceridemia, hypokalemia Nervous system disorders: Peripheral neuropathy, paresthesia, hypoesthesia, headache, dizziness Eye disorders: Visual disturbances, including vision blurred and diplopia Ear and labyrinth disorders: Transient or permanent hearing loss, tinnitus Cardiac disorders: Congestive heart failure Respiratory, thoracic and mediastinal disorders: Pulmonary edema Gastrointestinal disorders: Abdominal pain, vomiting, dyspepsia, nausea, diarrhea, constipation, dysgeusia Hepato-biliary disorders: Serious hepatotoxicity (including some cases of fatal acute liver failure), hepatitis, reversible increases in hepatic enzymes Skin and subcutaneous tissue disorders: Toxic epidermal necrolysis, Stevens-Johnson syndrome, exfoliative dermatitis, leukocytoclastic vasculitis, erythema multiforme, alopecia, photosensitivity, rash, urticaria, pruritus Musculoskeletal and connective tissue disorders: Myalgia, arthralgia Renal and urinary disorders: Urinary incontinence, pollakiuria Reproductive system and breast disorders: Menstrual disorders, erectile dysfunction General disorders and administration site conditions: Peripheral edema -
7 DRUG INTERACTIONS
7.1 Effects of ONMEL on Other Drugs
Itraconazole and its major metabolite, hydroxy-itraconazole, are strong inhibitors of the cytochrome P450 3A4 isoenzyme system (CYP3A4). Therefore, concomitant administration of ONMEL and certain drugs metabolized by the cytochrome CYP3A4 may result in increased plasma concentrations of those drugs due to decreased elimination, leading to potentially serious and/or life-threatening adverse events. Itraconazole is also an inhibitor of P-glycoprotein (P-gp) transporter and may result in increased plasma concentrations of drugs whose gastrointestinal absorption is regulated by P-gp. Whenever possible, plasma concentrations of these drugs should be monitored, and dosage adjustments made after concomitant ONMEL therapy is initiated. When appropriate, clinical monitoring for signs or symptoms of increased or prolonged pharmacologic effects is advised. Upon discontinuation, itraconazole plasma concentrations decline gradually (especially in patients with hepatic cirrhosis or in those receiving CYP3A4 inhibitors). This is particularly important when initiating therapy with drugs whose metabolism is affected by itraconazole.
7.2 Effects of Other Drugs on ONMEL
Inducers of CYP3A4 may decrease the plasma concentrations of itraconazole. ONMEL may not be effective in patients concomitantly taking ONMEL and one of these drugs. Therefore, administration of these drugs with ONMEL is not recommended.
Inhibitors of CYP3A4 may increase the plasma concentrations of itraconazole. Patients who must take ONMEL concomitantly with one of these drugs should be monitored closely for signs or symptoms of increased or prolonged pharmacologic effects of ONMEL.
Table 3. Selected Drugs that altered or are predicted to alter the plasma concentration of itraconazole or have their plasma concentration altered by ONMEL * - * This list is not all-inclusive.
- † For information on parenterally administered midazolam, see the Benzodiazepine paragraph below.
Drug plasma concentration increased by itraconazole Antiarrhythmics digoxin, dofetilide, quinidine, disopyramide Anticonvulsants carbamazepine Anti-HIV Agents indinavir, ritonavir, saquinavir, maraviroc Antineoplastics busulfan, docetaxel, vinca alkaloids Antipsychotics pimozide Benzodiazepines alprazolam, diazepam, midazolam, † triazolam Calcium Channel Blockers dihydropyridines (including nisoldipine and felodipine), verapamil Gastrointestinal Motility Agents cisapride HMG CoA-Reductase Inhibitors atorvastatin, cerivastatin, lovastatin, simvastatin Immunosuppressants Cyclosporine, tacrolimus, sirolimus Oral Hypoglycemics oral hypoglycemics (repaglinide) Opiate Analgesics fentanyl, levacetylmethadol (levomethadyl), methadone Polyene Antifungals amphotericin B Other ergot alkaloids, halofantrine, alfentanil, buspirone, methylprednisolone, budesonide, dexamethasone, fluticasone, warfarin, cilostazol, eletriptan, fexofenadine, loperamide Decrease plasma concentration of itraconazole Anticonvulsants carbamazepine, phenobarbital, phenytoin Anti-HIV Agents nevirapine, efavirenz Antimycobacterials isoniazid, rifabutin, rifampin Gastric Acid Suppressors/Neutralizers antacids, H 2-receptor antagonists, proton pump inhibitors Increase plasma concentration of itraconazole Macrolide Antibiotics clarithromycin, erythromycin Anti-HIV Agents indinavir, ritonavir Table 4. Selected Drugs that are contraindicated for use with itraconazole * - * This list is not all-inclusive.
- † For information on parenterally administered midazolam, see the Benzodiazepine paragraph below.
Antipsychotics pimozide Antiarrhythmics dofetilide, quinidine Benzodiazepines oral midazolam †, triazolam Calcium Channel Blockers Nisoldipine, felodipine Ergot Alkaloids dihydroergotamine, ergotamine, ergometrine (ergonovine), methylergometrine (methylergonovine) Gastrointestinal Motility Agents cisapride HMG CoA-Reductase Inhibitors lovastatin, simvastatin Opiate Analgesics levacetylmethadol (levomethadyl), methadone Antiarrhythmics
The Class IA antiarrhythmic, quinidine and class III antiarrhythmic, dofetilide are known to prolong the QT interval. Co-administration of quinidine or dofetilide with itraconazole may increase plasma concentrations of quinidine or dofetilide, which could result in serious cardiovascular events. Therefore, concomitant administration of ONMEL and quinidine or dofetilide is contraindicated. [See Boxed Warning, Contraindications (4), and Warnings and Precautions (5).]
The Class IA antiarrhythmic, disopyramide has the potential to increase the QT interval at high plasma concentrations. Caution is advised when ONMEL and disopyramide are administered concomitantly.
Concomitant administration of digoxin and itraconazole has led to increased plasma concentrations of digoxin via inhibition of P-glycoprotein.
Anticonvulsants
Carbamazepine, phenobarbital, and phenytoin are all inducers of CYP3A4. Reduced plasma concentrations of itraconazole were reported when itraconazole was administered concomitantly with phenytoin. Although interactions with carbamazepine and phenobarbital have not been studied, concomitant administration of ONMEL and these drugs would be expected to result in decreased plasma concentrations of itraconazole. In addition, in vivo studies have demonstrated an increase in plasma carbamazepine concentrations in subjects concomitantly receiving ketoconazole. Although there are no data regarding the effect of itraconazole on carbamazepine metabolism, because of the similarities between ketoconazole and itraconazole, concomitant administration of ONMEL and carbamazepine may inhibit the metabolism of carbamazepine.
Anti-HIV Agents
Non-nucleoside Reverse Transcriptase Inhibitors (NNRTI) such as nevirapine and efavirenz are inducers of CYP3A4. Human pharmacokinetic studies have shown that efavirenz, when concomitantly administered with itraconazole, greatly decreased serum concentrations of itraconazole and hydroxyl-itraconazole. Concomitant use of ONMEL and efavirenz is not recommended.
In vivo studies have shown that nevirapine induces the metabolism of ketoconazole, significantly reducing the bioavailability of ketoconazole. Studies involving nevirapine and itraconazole have not been conducted. However, because of the similarities between ketoconazole and itraconazole, concomitant administration of ONMEL and nevirapine is not recommended.
Concomitant administration of ONMEL and protease inhibitors metabolized by CYP3A4, such as indinavir, ritonavir, and saquinavir, may increase plasma concentrations of these protease inhibitors. In addition, concomitant administration of ONMEL and indinavir and ritonavir (but not saquinavir) may increase plasma concentrations of itraconazole. Caution is advised when ONMEL and protease inhibitors must be given concomitantly.
Concomitant administration of ONMEL and maraviroc has been reported to increase plasma concentration of maraviroc. The dose of maraviroc should be decreased to 150 mg twice daily when given in combination with itraconazole.
Antimycobacterials
Drug interaction studies have demonstrated that plasma concentrations of azole antifungal agents and their metabolites, including itraconazole and hydroxyitraconazole, were significantly decreased when these agents were given concomitantly with rifabutin or rifampin. In vivo data suggest that rifabutin is metabolized in part by CYP3A4. ONMEL may inhibit the metabolism of rifabutin. Although no formal study data are available for isoniazid, similar effects should be anticipated. Therefore, the efficacy of ONMEL could be substantially reduced if given concomitantly with one of these agents and co-administration is not recommended.
Antipsychotics
Pimozide is known to prolong the QT interval and is partially metabolized by CYP3A4. Co-administration of pimozide with itraconazole could result in serious cardiovascular events. Therefore, concomitant administration of ONMEL and pimozide is contraindicated. [See Boxed Warning, Contraindications (4), and Warnings and Precautions (5).]
Increases in plasma aripiprazole concentrations have been demonstrated in subjects concomitantly receiving ketoconazole, requiring a reduction of the aripiprazole dose. Because of the similarities between ketoconazole and itraconazole, a similar dose reduction for aripiprazole is recommended when patients concomitantly receive itraconazole and aripiprazole.
Benzodiazepines
Concomitant administration of itraconazole and alprazolam, diazepam, oral midazolam, or triazolam could lead to increased plasma concentrations of these benzodiazepines. Increased plasma concentrations could potentiate and prolong hypnotic and sedative effects. Concomitant administration of ONMEL and oral midazolam or triazolam is contraindicated. [See Contraindications (4), and Warnings and Precautions (5).] If midazolam is administered parenterally, special precaution and patient monitoring is required since the sedative effect may be prolonged.
Calcium Channel Blockers
Calcium channel blockers can have a negative inotropic effect which may be additive to those of itraconazole; itraconazole can inhibit the metabolism of calcium channel blockers such as dihydropyridines (e.g., nifedipine, nisoldipine, and felodipine) and verapamil. Therefore, caution should be used when co-administering itraconazole and calcium channel blockers due to an increased risk of CHF.
Concomitant administration of ONMEL and nisoldipine results in clinically significant increases in nisoldipine plasma concentrations, which cannot be managed by dosage reduction, therefore the concomitant administration of ONMEL and nisoldipine is contraindicated. A clinical study showed that felodipine exposure was increased by co-administration of itraconazole, resulting in approximately 6-fold increase in the AUC and 8-fold increase in the C max. The concomitant use of ONMEL and felodipine is contraindicated. [See Contraindications (4), Warnings and Precautions (5), Drug Interactions (7), and Clinical Pharmacology (12).]
Edema has been reported in patients concomitantly receiving itraconazole and dihydropyridine calcium channel blockers. Appropriate dosage adjustment may be necessary.
Gastric Acid Suppressors/Neutralizers
Reduced plasma concentrations of itraconazole were reported when administered concomitantly with H 2-receptor antagonists. Studies have shown that absorption of itraconazole is impaired when gastric acid production is decreased. ONMEL should be administered with a cola beverage if the patient has achlorhydria or is taking H 2-receptor antagonists or other gastric acid suppressors. It is advised that antacids be administered at least 1 hour before or 2 hours after administration of ONMEL. In a clinical study, when itraconazole capsules were administered with omeprazole (a proton pump inhibitor), the bioavailability of itraconazole was significantly reduced.
Gastrointestinal Motility Agents
Co-administration of itraconazole with cisapride can elevate plasma cisapride concentrations, which could result in serious cardiovascular events. Therefore, concomitant administration of ONMEL with cisapride is contraindicated. [See Boxed Warning, Contraindications (4), and Warnings and Precautions (5).]
3-Hydroxy-3-Methyl-Glutaryl CoA-Reductase Inhibitors
Human pharmacokinetic data suggest that itraconazole inhibits the metabolism of atorvastatin, cerivastatin, lovastatin, and simvastatin, which may increase the risk of skeletal muscle toxicity, including rhabdomyolysis. Concomitant administration of ONMEL with 3-Hydroxy-3-Methyl-Glutaryl (HMG) CoA-Reductase inhibitors, such as lovastatin and simvastatin, is contraindicated. [See Contraindications (4), and Warnings and Precautions (5).]
Immunosuppressants
Concomitant administration of ONMEL and cyclosporine or tacrolimus has led to increased plasma concentrations of these immunosuppressants. Similarly, concomitant administration of ONMEL and sirolimus could increase plasma concentrations of sirolimus.
Monitoring of blood concentrations of cyclosporine, tacrolimus, or sirolimus are recommended when ONMEL are co-administered with these immunosuppressants and appropriate dosage adjustments should be made.
Macrolide Antibiotics
Erythromycin and clarithromycin are known inhibitors of CYP3A4 (See Table 3) and may increase plasma concentrations of itraconazole.
Oral Hypoglycemic Agents
Severe hypoglycemia has been reported in patients concomitantly receiving azole antifungal agents and oral hypoglycemic agents. A human pharmacokinetic study showed that co-administration with itraconazole and a single dose of repaglinide (on the third day of a regimen of 200 mg initial dose, twice-daily 100 mg itraconazole) resulted in a 1.4-fold higher repaglinide AUC. Blood glucose concentrations should be carefully monitored when ONMEL and oral hypoglycemic agents are co-administered.
Polyenes Antifungal Agents
Prior treatment with itraconazole, like other azoles, may reduce or inhibit the activity of polyenes such as amphotericin B. However, the clinical significance of this drug effect has not been clearly defined.
Opiate Analgesics
Levacetylmethadol (levomethadyl) and methadone are known to prolong the QT interval and are metabolized by CYP3A4. Co-administration of methadone or levacetylmethadol with itraconazole could result in serious cardiovascular events. Therefore, concomitant administration of ONMEL and methadone or levacetylmethadol are contraindicated.
Fentanyl plasma concentrations could be increased or prolonged by concomitant use of itraconazole and may cause potentially fatal respiratory depression.
In vitro data suggest that alfentanil is metabolized by CYP3A4. Administration with itraconazole may increase plasma concentrations of alfentanil.
Other
- Elevated concentrations of ergot alkaloids can cause ergotism, i.e., a risk for vasospasm potentially leading to cerebral ischemia and/or ischemia of the extremities. Concomitant administration of ergot alkaloids such as dihydroergotamine, ergometrine (ergonovine), ergotamine and methylergometrine (methylergonovine) with ONMEL is contraindicated.
- Halofantrine has the potential to prolong the QT interval at high plasma concentrations. Caution is advised when ONMEL and halofantrine are administered concomitantly.
- Human pharmacokinetic data suggest that concomitant administration of itraconazole and buspirone results in significant increases in plasma concentrations of buspirone.
- Itraconazole may inhibit the metabolism of certain glucocorticosteroids such as budesonide, dexamethasone, fluticasone and methylprednisolone.
- Itraconazole enhances the anticoagulant effect of coumarin-like drugs, such as warfarin.
- Cilostazol and eletriptan are CYP3A4 metabolized drugs that should be used with caution when co-administered with ONMEL.
- Co-administration of itraconazole with meloxicam decreased peak plasma concentrations and the exposure of meloxicam by 64% and 37%, respectively. Monitor patients for responses to meloxicam when itraconazole is concomitantly administered and dose adjustment should be considered if warranted.
- Co-administration of itraconazole with fexofenadine increased the peak plasma concentration and the total exposure of fexofenadine by approximately 3-fold and augmented its anti-histamine effects.
- Co-administration of itraconazole with loperamide increased peak plasma concentrations of loperamide by 3-fold and the total exposure by 3.9-fold. In addition, itraconazole is an inhibitor of P-glycoprotein and may inhibit the transport of loperamide out of the brain, leading to elevated concentrations of loperamide in the brain. Patients should be monitored for signs and symptoms of loperamide overdose, such as CNS depression, including drowsiness, dizziness and respiratory depression, and a dose or dosing frequency should be adjusted as necessary.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic effects. Pregnancy Category C
There are no adequate and well-controlled clinical trials in the pregnant women with itraconazole. However, cases of congenital abnormalities have been reported with itraconazole drug products in post-marketing reports. Therefore, ONMEL should not be administered to pregnant women, women planning pregnancy, or women of child bearing potential unless these onychomycosis patients are using effective contraception measures to prevent pregnancy. Effective contraceptive measures should continue throughout the treatment period and for two months thereafter. ONMEL should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Itraconazole produced a significant dose-related increase in maternal toxicity, embryotoxicity, and teratogenicity in rats at dose levels of 40-160 mg/kg/day (2-10 times the maximum recommended human dose [MRHD], based on mg/m 2/day comparisons), and in mice at 80 mg/kg/day (2 times MRHD, based on mg/m 2/day comparisons). Teratogenic changes in rats included major skeletal defects; encephalocele and/or macroglossia developed in mice.
8.3 Nursing Mothers
Itraconazole is excreted in human milk; therefore, the expected benefits of ONMEL therapy for the mother should be weighed against the potential risk from exposure of itraconazole to the infant.
8.4 Pediatric Use
The safety and effectiveness of ONMEL in pediatric patients have not been established. No pharmacokinetic data on ONMEL are available in children.
8.5 Geriatric Use
ONMEL was evaluated in 42 of 593 subjects (7.1%) greater than 65 years of age.
Transient or permanent hearing loss has been reported in elderly patients receiving treatment with itraconazole. Several of these reports included concurrent administration of quinidine which is contraindicated. [See Boxed Warning, Contraindications (4), Drug Interactions (7), and Warnings and Precautions (5).] Itraconazole should be used with care in elderly patients. [See Warnings and Precautions (5).]
8.6 Renal Impairment
Limited data are available on the use of oral itraconazole in patients with renal impairment. Caution should be exercised when ONMEL is administered to patients with renal impairment. [See Clinical Pharmacology (12.5) and Dosage and Administration (2).]
8.7 Hepatic Impairment
Limited data are available on the use of oral itraconazole in patients with hepatic impairment. Caution should be exercised when ONMEL is administered to patients with hepatic impairment. [See Clinical Pharmacology (12.5) and Dosage and Administration (2).]
- 10 OVERDOSAGE
-
11 DESCRIPTION
ONMEL (itraconazole) is a synthetic triazole antifungal agent for oral use. Itraconazole is a 1:1:1:1 racemic mixture of four diastereomers (two enantiomeric pairs), each possessing three chiral centers. It may be represented by the following structural formula and nomenclature:
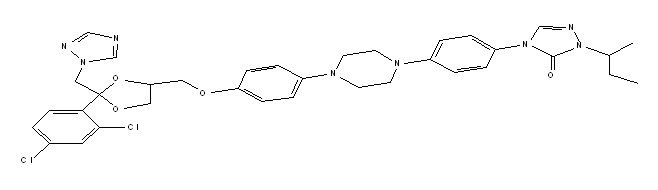
(±)- cis-4-[4-[4-[4[[2-(2,4-dichlorophenyl)-2-(1 H-1,2,4-triazol-1-ylmethyl)-1,3-dioxolane-4-yl]methoxy]phenyl]-1-piperazinyl]phenyl]-2,4-dihydro-2-(1-methylpropyl)-3 H-1,2,4-triazol-3-one
Itraconazole has a molecular formula of C 35H 38Cl 2N 8O 4 and a molecular weight of 705.64. It is a white to slightly yellowish powder. It is insoluble in water, very slightly soluble in alcohols, and freely soluble in dichloromethane. It has a pKa of 3.70 (based on extrapolation of values obtained from methanolic solutions) and a log (n-octanol/water) partition coefficient of 5.66 at pH 8.1.
Each ONMEL is formulated for melt extrusion technology and contains 200 mg of itraconazole and the following inactive ingredients: colloidal silicon dioxide, crospovidone, hydrogenated vegetable oil, hypromellose, lactose, microcrystalline cellulose, magnesium stearate, propylene glycol, talc, and titanium dioxide.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Itraconazole, an azole, is an antifungal agent [ See Clinical Pharmacology (12) and Microbiology (12.4)].
12.3 Pharmacokinetics
The oral bioavailability of itraconazole is increased when ONMEL is taken with a FDA standard high-fat meal. The pharmacokinetic parameters of itraconazole and hydroxy-itraconazole after administration of one ONMEL to 9 male and 9 female healthy subjects in fasting and in fed conditions are presented in the table below:
Table 5: Pharmacokinetic Parameters Following a Single Dose of ONMEL (mean ± SD) * Itraconazole Hydroxy-itraconazole Fed Fasted Fed Fasted - * Drug given after FDA standard high-fat breakfast
- † mean ± standard deviation
C max (ng/mL) 213 ± 117 † 162 ± 107 332 ± 118 264 ± 109 T max (hours) 4.6 ± 2.2 2.9 ± 0.8 5.7 ± 2.6 3.4 ± 0.8 AUC 0-∞ (μg.h/mL) 3.34 ± 1.98 2.27 ± 1.44 7.05 ± 3.94 4.58 ± 2.80 The steady-state pharmacokinetics of itraconazole and hydroxy-itraconazole were analyzed after oral dosing of 16 healthy volunteers with one ONMEL following a moderate-fat breakfast once daily for 14 days in an open-label study. Mean maximum plasma levels of itraconazole and hydroxy-itraconazole increased from Day 1 to Day 14 by approximately 6- and 4-fold, respectively. The respective pharmacokinetic parameters from this study are reflected in the table below:
Table 6: Pharmacokinetic Parameters Following Multiple Doses of ONMEL (mean ± SD) Taken with Moderate-fat Breakfasts * Statistic Day Itraconazole
N=16Hydroxy-itraconazole
N=16- * Meal containing approximately 500 calories, 30% of which were derived from fat.
C max (ng/mL) Mean (SD) 1 116.8 (43.34) 221.7 (69.21) 14 658.1 (362.16) 974.2 (479.92) AUC 0-24 (ng*h/mL) Mean (SD) 1 905.09 (384.239) 2538.33 (1057.872) 14 9046.81 (5320.516) 19054.95 (10443.214) T max (h) Median
(Min-Max)1 4.00 (2.00-5.00) 4.00 (2.00-5.00) 14 4.00 (1.00-24.00) 4.00 (3.00-24.00) T 1/2 (h) Mean (SD) 14 36.84 (10.378) 20.06 (6.998) In a 2-period, open-label, randomized, cross-over, pivotal bioequivalence study to assess the comparative bioavailability of the ONMEL and a marketed 100-mg itraconazole capsule, 28 male and 28 female healthy subjects were given as a single dose, 200 mg of itraconazole immediately after a moderate-fat breakfast ( same caloric and fat contents as in the table above). Fifty-two subjects were included in the final analysis.
The C max of the ONMEL was comparable to that of the 2 itraconazole 100-mg capsules while AUC t and AUC ∞ were about 15% higher with the ONMEL.
In another 2-period, open-label, randomized, cross-over, pivotal bioequivalence study, 28 male and 28 female healthy subjects were given one ONMEL or two 100-mg itraconazole capsules following the FDA standard high-fat breakfast. The C max and AUC ∞ of the ONMEL were 20 and 30% lower, respectively, than those of two itraconazole 100-mg capsules. Overall, the inter-subject variability was high and coefficient of variances (CV) for AUCs in the above two studies were 44-66%.
Itraconazole is metabolized predominately by the cytochrome P450 3A4 isoenzyme system (CYP3A4), resulting in the formation of several metabolites. Hydroxyitraconazole, the major metabolite, has in vitro antifungal activity comparable to itraconazole. Results of a pharmacokinetics study suggest that itraconazole may undergo saturable metabolism with multiple dosing. Based on an oral dose, fecal excretion of the parent drug varies between 3-18% of the dose. Itraconazole is excreted mainly as inactive metabolites in the urine (35%) and feces (54%) within one week of an oral dose. No single excreted metabolite represents more than 5% of a dose. The plasma protein binding of itraconazole has been reported to be 99.8% and that of hydroxy-itraconazole is 99.5%. [See Contraindications (4).]
12.4 Microbiology
Mechanism of Action
Itraconazole inhibits the cytochrome P450-dependent synthesis of ergosterol, which is a vital component of fungal cell membranes.
Activity In Vitro
Itraconazole exhibits in vitro activity against Trichophyton rubrum and Trichophyton mentagrophytes.
Resistance
Isolates from several fungal species with decreased susceptibility to itraconazole have been isolated from patients receiving prolonged therapy.
Several in vitro studies have reported that some fungal clinical isolates with reduced susceptibility to one azole antifungal agent may also be less susceptible to other azole derivatives. The finding of cross-resistance is dependent on a number of factors, including the species evaluated, its clinical history, the particular azole compounds compared, and the type of susceptibility test that is performed. The relevance of these in vitro susceptibility data to clinical outcome remains to be elucidated.
Special Populations
Renal Insufficiency
Limited data are available on the use of oral itraconazole in patients with renal impairment. A pharmacokinetic study using a single 200-mg dose of itraconazole was conducted in three groups of patients with renal impairment (uremia: n=7; hemodialysis: n=7; and continuous ambulatory peritoneal dialysis: n=5). In uremic subjects with a mean creatinine clearance of 13 mL/min. × 1.73 m 2, the exposure, based on AUC, was slightly reduced compared with normal population parameters. The study did not demonstrate any significant effect of hemodialysis or continuous ambulatory peritoneal dialysis on the pharmacokinetics of itraconazole (T max, C max, and AUC 0-8). Plasma concentration-versus-time profiles showed wide intersubject variation in all three groups. Caution should be exercised when the drug is administered in this population. [See Warnings and Precautions (5) and Dosage and Administration (2).]
Hepatic Insufficiency
Itraconazole is predominantly metabolized in the liver. Patients with impaired hepatic function should be carefully monitored when taking itraconazole. A pharmacokinetic study using a single oral 100 mg dose of itraconazole was conducted in 6 healthy and 12 cirrhotic subjects. A statistically significant reduction in mean C max (47%) and a twofold increase in the elimination half-life (37 ± 17 hours vs. 16 ± 5 hours) of itraconazole were noted in cirrhotic subjects compared with healthy subjects. However, overall exposure to itraconazole, based on AUC, was similar in cirrhotic patients and in healthy subjects. The prolonged elimination half-life of itraconazole observed in the single oral dose clinical trial with itraconazole in cirrhotic patients should be considered when deciding to initiate therapy with other medications metabolized by CYP3A4. Data are not available in cirrhotic patients during long-term use of itraconazole. [See Boxed Warning, Contraindications (4), Warnings and Precautions (5), and Dosage and Administration (2).]
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No carcinogenicity, mutagenicity, or impairment of fertility studies were conducted with ONMEL.
Itraconazole did not exhibit any carcinogenic potential in mice receiving oral doses up to 80 mg/kg/day (2 times MRHD, based on mg/m 2/day comparisons) for 23 months. A slightly increased incidence of soft tissue sarcoma was observed in male rats administered 25 mg/kg/day (1.3 times MRHD, based on mg/m 2/day comparisons). These tumors may have been related to hypercholesterolemia caused by chronic treatment with itraconazole in rats; hypercholesterolemia is not observed with such treatment in dogs or humans. Compared to untreated controls, female rats receiving 50 mg/kg/day (2.5 times MRHD, based on mg/m 2/day comparisons) had a statistically insignificant increase in squamous cell carcinoma in lungs (2/50), an uncommon tumor in rats.
Itraconazole did not exhibit any mutagenic or genotoxic effects when evaluated in a DNA repair test (unscheduled DNA synthesis) in primary rat hepatocytes, in Ames tests (6 Salmonella strains and E. coli), in the mouse lymphoma gene mutation test, in a sex-linked recessive lethal mutation ( Drosophila melanogaster) test, in chromosome aberration test (human lymphocytes), in a cell transformation assay (C3H/10T ½ C18 mouse embryo fibroblasts), in a dominant lethal mutation test in male and female mice, and in micronucleus tests in mice and rats.
Itraconazole did not affect the fertility in male or female rats treated with oral doses up to 40 mg/kg/day (2 times MRHD, based on mg/m 2/day comparisons); however, parental toxicity occurred at this dosage. More severe parental toxicity was observed at 160 mg/kg/day (10 times MRHD, based on mg/m 2/day comparisons).
-
14 CLINICAL STUDIES
The efficacy of ONMEL for the treatment of onychomycosis of the toenail was examined in a randomized, multi-center, placebo-controlled, third-party blinded trial comparing ONMEL to two 100 mg itraconazole capsules and placebo tablets.
In the clinical study, 791 subjects with diagnosis of distal and/or lateral subungual onychomycosis were randomized to ONMEL (N= 593) or placebo tablets (N= 198) once daily for 12 consecutive weeks. The median age of subjects enrolled in the trial was 48 years and 75% were males. At baseline, 95.1% of subjects had onychomycosis due to T. rubrum with a baseline global severity score of 'Moderate' which was defined as a target toenail involvement ≤50% dystrophy and/or discoloration with clear evidence of subungual hyperkeratosis and/or onycholysis.
The primary endpoint was the proportion of subjects with a Complete Cure at Week 52, nine months after completion of study medication. A Complete Cure was defined as both a Clinical Cure (no evidence of onychomycosis in target nail; normal nail unit without subungual hyperkeratosis or onycholysis) and Mycological Cure (negative KOH and negative culture). The following table illustrates the study results for ONMEL and Placebo:
Table 7: Primary Efficacy Results at Week 52 Endpoint ONMEL
N=593Placebo
N=198- * Complete Cure defined as Clinical Cure (no evidence of onychomycosis in target nail; normal nail unit without subungual hyperkeratosis or onycholysis) and Mycological Cure (negative KOH and negative culture)
Complete Cure * 22.3% 1.0% The Mycologic Cure rate was 44% and the Clinical Cure rate was 26% for subjects treated with ONMEL. Comparatively, the Mycological Cure rate was 6% and the Clinical Cure rate was 3% for subjects treated with Placebo Tablets.
Efficacy results comparing ONMEL to 200 mg of itraconazole capsules (two 100 mg capsules) were similar.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
ONMEL is available containing 200 mg of itraconazole, as a white to slightly grey, oblong, biconvex tablet engraved with "BARRIER" on one side and "It 200" on the other side. Each carton (NDC: 0259-1420-28) contains two blister cards of 14 tablets each (NDC: 0259-1420-14).
- 17 PATIENT COUNSELING INFORMATION
- SPL UNCLASSIFIED SECTION
-
PATIENT PACKAGE INSERT
FDA-Approved Patient Labeling
ONMEL (itraconazole)
Read this Patient Information before you start using ONMEL and each time you get a refill. There may be new information. This information does not take the place of talking with your doctor about your medical condition or treatment.
What is the most important information I should know about ONMEL?
ONMEL can cause serious life-threatening side effects, including:
-
Heart Failure.
Do not take ONMEL if you have had heart failure, including congestive heart failure.
Stop taking ONMEL and call your doctor right away if you have any of these symptoms of congestive heart failure:
- shortness of breath
- swelling of your feet, ankles, or legs
- sudden weight gain
- increased tiredness
- coughing up white or pink phlegm
- fast heartbeat
- waking up at night more than normal for you
-
Serious cardiovascular effects. Do not take ONMEL if you also take the following medicines:
- cisapride (Propulsid)
- pimozide (Orap)
- quinidine (Quindine Gluconate, Quindine
- Sulfate)
- dofetilide (Tikosyn)
- levomethadyl (Oralaam)
- midazolam (Versed)
- felodipine, nisoldipine (Lexxel, Plendil, Sular)
- triazolam (Halcion)
- lovastatin (Mevacor, Advicor, Altoprev)
- simvastatin (Zocor, Simcor, Vytorin)
- ergot alkaloids (Migranal, Ergonovine, Cafergot, Methergine, Dihydroergotamine Mesylate, Methylergonovine)
- methadone (Dolophine)
This is not a complete list of medicines that can interact with ONMEL.
- Before taking ONMEL, tell your doctor about all the medicine you take, including prescription and non-prescription medicines, vitamins, and herbal supplements.
- Before you start any new medicine, ask your doctor or pharmacist if it is safe to take it with ONMEL.
What is ONMEL?
ONMEL is a prescription medicine used to treat fungal infections of the toenails. It is not known if ONMEL is safe and effective in children under the age of 18.
Do not take ONMEL if you:
- have or had heart failure, including congestive heart failure.
- Take certain medicines. See " What is the most important information I should know about ONMEL?"
- are pregnant or plan to become pregnant. ONMEL can harm your unborn baby. Talk to your doctor if you are pregnant or plan to become pregnant.
- have ever had an allergic reaction to itraconazole or any of the other ingredients in ONMEL. Ask your doctor or pharmacist for a list of these ingredients.
What should I tell my doctor before taking ONMEL?
Before taking ONMEL, tell your doctor if you:
- have or had heart, lung, liver or kidney problems
- have any other medical conditions
- are pregnant or planning to become pregnant. See " Who should not take ONMEL?". Females who can become pregnant should use effective birth control during treatment with ONMEL and for 2 months after you stop treatment with ONMEL. Talk to your doctor about the type of birth control that is best for you while taking ONMEL.
- are breast-feeding or plan to breast-feed. ONMEL can pass into your breastmilk and may harm your baby. Talk to your doctor about the best way to feed your baby if you take ONMEL.
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements.
Taking ONMEL with certain other medicines could lead to serious or life-threatening medical problems.
- See " What is the most important information I should know about ONMEL?"
- Fentanyl. Taking fentanyl, a strong opioid narcotic main medicine with ONMEL could cause serious breathing problems that can lead to death.
Talk to your doctor or pharmacist before you start any new medicine. They can tell you if it is safe to take ONMEL with your other medicines.
Know the medicines you take. Keep a list of your medicines and show it to your doctor and pharmacist when you get a new medicine.
How should I take ONMEL?
Take ONMEL exactly as prescribed by your doctor. Be sure to finish all of your ONMEL as prescribed by your doctor.
- ONMEL comes in a 14 tablet blisterpack container.
- Take ONMEL with a full meal at the same time each day.
- Your doctor should do blood tests to check your liver function before you start and while you take ONMEL, especially if you have liver problems.
- If you forget to take or miss doses of ONMEL, skip that dose and take the next dose at your regular time. Do not make up missed doses.
- If you take too many ONMEL, call your local poison control center or go to the nearest hospital emergency room right away.
What are the possible side effects of ONMEL?
ONMEL can cause serious side effects, including:
- See " What is the most important information I should know about ONMEL?"
-
liver failure and death.
Stop taking ONMEL and call your doctor right away if you have symptoms of liver failure including:
- unusually tired
- lose your appetite
- nausea
- abdominal pain
- vomiting
- yellow change in the color of your skin or eyes
- dark colored urine
- pale stools (bowel movements)
- nerve damage (neuropathy). Call your doctor right away if you have tingling or numbness in your hands or feet. You may need to stop taking ONMEL if this happens.
- hearing loss. Hearing loss can happen for a short time or permanently in some people who take ONMEL with other medications. Stop taking ONMEL and call your doctor right away if you have any changes in your hearing.
Common side effects of ONMEL include:
- increased liver enzyme in blood test results
- upper respiratory infection or cold (runny nose, cough and sneeze)
- urinary tract infection (burning and painful urination)
- stomach pain
- diarrhea
- nausea
- headache
- tiredness
- throat pain
- back pain
These are not all of the possible side effects of ONMEL. Tell your doctor if you any side effect that bothers you or that does not go away. For more information, ask your doctor.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store ONMEL?
- Store at controlled room temperature between 59° to 77°F (15° to 25°C).
- Keep ONMEL away from light and moisture.
- Keep ONMEL and all medicines out of reach of children.
General Information:
Medicines are sometimes prescribed for conditions that are not mentioned in Patient Information leaflets. Do not use ONMEL for a condition for which it was not prescribed. Do not give ONMEL to other people, even if they have the same symptoms that you have. It may harm them.
This leaflet summarizes the most important information about ONMEL. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about ONMEL that is written for health professionals.
For more information about ONMEL call 1-877-743-8454.
What are the ingredients in ONMEL?
Active ingredient: itraconazole
Inactive ingredients: colloidal silicon dioxide, crospovidone, hydrogenated vegetable oil, hypromellose, lactose, magnesium stearate, microcrystalline cellulose, propylene glycol, talc, and titanium dioxide.
The following are registered trademarks of their respective manufacturers:
Dolophine ® (Roxane Laboratories, Inc), Mevacor ® (Merck & Co., Inc.), Advicor ® (Kos Pharmaceuticals, Inc.), Altocor™ (Andrx Laboratories), Zocor ® (Merck & Co., Inc.), Halcion ® (Pharmacia), Versed ® (Roche Pharmaceuticals), Cardioquin ® (The Purdue Frederick Company), Quinaglute ® (Berlex Laboratories), Quinidex ® (A.H. Robins), Tikosyn™ (Pfizer, Inc.), Propulsid ® (Janssen Pharmaceutica Products, L.P.), Orlaam ® (Roxane Laboratories), Migranal ® (Xcel Pharmaceuticals), Ergonovine (PDRX Pharmaceuticals), Cafergot ® (Novartis Pharmaceuticals Corporation), Methergine ® (Novartis Pharmaceuticals Corporation), Orap ® (Gate Pharmaceuticals), and Sular ® (First Horizon Pharmaceutical Corporation)
What happens if I have a fungal nail infection?
Anyone can have a fungal nail infection, but it is usually found in adults. When a fungus infects the nail, the infected part of the nail may turn yellow or brown. If not treated, the fungus may spread, and more of the nail may change color, may become thick or brittle, and the tip of the nail may become raised. In some patients, this can cause pain and discomfort.
Manufactured by:
Sanico N.V.
2300 Turnhout, BelgiumManufactured for
Merz Pharmaceuticals, LLC
4215 Tudor Lane
Greensboro, NC 274105011889
10/2012
ONM:3PIL
-
Heart Failure.
Do not take ONMEL if you have had heart failure, including congestive heart failure.
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 28 Tablet Carton
NDC: 0259-1420-28
Rx Only
MERZ
2 Blister Cards
28 Tablets Total
Each Blister card contains
14 TabletsONMEL™
(itraconazole) Tablets 200 mg
-
INGREDIENTS AND APPEARANCE
ONMEL
itraconazole tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0259-1420 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ITRACONAZOLE (UNII: 304NUG5GF4) (ITRACONAZOLE - UNII:304NUG5GF4) ITRACONAZOLE 200 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSPOVIDONE (15 MPA.S AT 5%) (UNII: 68401960MK) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) MAGNESIUM STEARATE (UNII: 70097M6I30) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color white (white to slightly grey) Score no score Shape FREEFORM (oblong) Size 19mm Flavor Imprint Code BARRIER;IT200 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0259-1420-28 2 in 1 CARTON 11/01/2012 11/30/2020 1 NDC: 0259-1420-14 14 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022484 11/01/2012 11/30/2020 Labeler - Merz Pharmaceuticals, LLC (126209282) Registrant - Sebela Pharmaceuticals Inc. (079104574) Establishment Name Address ID/FEI Business Operations Sanico N.V. 282896711 manufacture(0259-1420)
Trademark Results [Onmel]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ONMEL 85014407 4429079 Live/Registered |
SEBELA IRELAND LIMITED 2010-04-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.