ZAFEMY- norelgestromin and ethinyl estradiol patch
ZAFEMY by
Drug Labeling and Warnings
ZAFEMY by is a Prescription medication manufactured, distributed, or labeled by AvKARE. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
ZAFEMY ® (norelgestromin and ethinyl estradiol transdermal system)
These highlights do not include all the information needed to use ZAFEMY safely and effectively. See full prescribing information for ZAFEMY.
ZAFEMY ® (norelgestromin and ethinyl estradiol transdermal system)
Initial U.S. Approval: 2001WARNING: CIGARETTE SMOKING AND SERIOUS CARDIOVASCULAR EVENTS and CONTRAINDICATED IN WOMEN WITH A BMI ≥ 30 kg/m 2
See full prescribing information for complete boxed warning.
RECENT MAJOR CHANGES
Warnings and Precautions ( 5.12) 11/2021
INDICATIONS AND USAGE
ZAFEMY ® is an estrogen/progestin combination hormonal contraceptive (CHC), indicated for the prevention of pregnancy in women with a BMI < 30 kg/m 2 for whom a combined hormonal contraceptive is appropriate. (1)
Limitations of Use: ZAFEMY may be less effective in preventing pregnancy in women at or above 198 lbs (90 kg). ( 1, 4, 14)
DOSAGE AND ADMINISTRATION
- ZAFEMY uses a 28-day (four-week) cycle. Apply a new patch to the upper outer arm, abdomen, buttock or back each week for three weeks (21 total days). Week Four is patch-free. (2.1, 2.3)
- Apply each new patch on the same day of the week. Wear only one patch at a time. (2.1)
- Do not cut or alter the patch in any way. (2.1)
DOSAGE FORMS AND STRENGTHS
Transdermal system: 150 mcg/day norelgestromin, USP and 35 mcg/day ethinyl estradiol, USP. (3)
CONTRAINDICATIONS
- At high risk of arterial or venous thromboembolic events (4)
- BMI ≥ 30 kg/m 2(4)
- Breast cancer or other estrogen- or progestin-sensitive cancer (4)
- Liver tumors or liver disease (4)
- Undiagnosed abnormal uterine bleeding (4)
- Pregnancy (4)
- Co-administration with Hepatitis C drug combinations containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir (4)
WARNINGS AND PRECAUTIONS
- Vascular risks: Stop ZAFEMY if a thrombotic event occurs. Stop at least 4 weeks before and through 2 weeks after major surgery. Start no earlier than 4 weeks after delivery, in women who are not breastfeeding. Consider cardiovascular risk factors before initiating in all women, particularly those over 35 years. (5.1)
- Liver disease: Discontinue ZAFEMY if jaundice occurs. (5.3)
- High blood pressure: Do not prescribe ZAFEMY for women with uncontrolled hypertension or hypertension with vascular disease. (5.5)
- Carbohydrate and lipid metabolic effects: Monitor prediabetic and diabetic women taking ZAFEMY. Consider an alternate contraceptive method for women with uncontrolled dyslipidemia. (5.7)
- Headache: Evaluate significant change in headaches and discontinue ZAFEMY if indicated. (5.8)
- Uterine bleeding: Evaluate irregular bleeding or amenorrhea. (5.9)
ADVERSE REACTIONS
The most frequent adverse reactions reported during clinical trials (≥ 5%) were breast symptoms, nausea/vomiting, headache, application site disorder, abdominal pain, dysmenorrhea, vaginal bleeding and menstrual disorders, and mood, affect and anxiety disorders. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact AvKARE at 1-855-361-3993 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Drugs or herbal products that induce certain enzymes (for example CYP3A4) may decrease the effectiveness of CHCs or increase breakthrough bleeding. Counsel patients to use a back-up or alternative method of contraception when enzyme inducers are used with CHCs. (7.1)
USE IN SPECIFIC POPULATIONS
- Nursing mothers: Not recommended; can decrease milk production. (8.3)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 4/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: CIGARETTE SMOKING AND SERIOUS CARDIOVASCULAR EVENTS and CONTRAINDICATED IN WOMEN WITH A BMI ≥ 30 kg/m2
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 How to Use ZAFEMY
2.2 How to Start Using ZAFEMY
2.3 How to Apply ZAFEMY
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Thromboembolic Disorders and Other Vascular Conditions
5.2 Ethinyl Estradiol Exposure
5.3 Liver Disease
5.4 Risk of Liver Enzyme Elevations with Concomitant Hepatitis C Treatment
5.5 High Blood Pressure
5.6 Gallbladder Disease
5.7 Carbohydrate and Lipid Metabolic Effects
5.8 Headache
5.9 Bleeding Irregularities
5.10 Hormonal Contraceptive Use Before or During Early Pregnancy
5.11 Depression
5.12 Malignant Neoplasms
5.13 Effect on Binding Globulins
5.14 Monitoring
5.15 Hereditary Angioedema
5.16 Chloasma
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Effects of Other Drugs on Combined Hormonal Contraceptives
7.2 Effects of Combined Hormonal Contraceptives on Other Drugs
7.3 Concomitant Use with HCV Combination Therapy – Liver Enzyme Elevation
7.4 Interference with Laboratory Tests
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
8.8 BMI and Weight Considerations
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, and Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Special Precautions for Storage and Disposal
17 PATIENT COUNSELING INFORMATION
17.1 General
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: CIGARETTE SMOKING AND SERIOUS CARDIOVASCULAR EVENTS and CONTRAINDICATED IN WOMEN WITH A BMI ≥ 30 kg/m2
-
Cigarette Smoking and Serious Cardiovascular Events
Cigarette smoking increases the risk of serious cardiovascular events from hormonal contraceptive use. This risk increases with age, particularly in women over 35 years of age, and with the number of cigarettes smoked. For this reason, CHCs, including ZAFEMY, are contraindicated in women who are over 35 years of age and smoke [see Contraindications (4) and Warnings and Precautions (5.1)] .
- Contraindicated in Women with a BMI ≥ 30 kg/m 2
ZAFEMY is contraindicated in women with a BMI ≥ 30 kg/m 2. The risk of VTE may be greater with ZAFEMY in women with a BMI > 30 kg/m 2 compared to women with a lower BMI [see Contraindications (4) and Warnings and Precautions (5.1)] .
-
Cigarette Smoking and Serious Cardiovascular Events
-
1 INDICATIONS AND USAGE
ZAFEMY ® is indicated for the prevention of pregnancy in women with a body mass index (BMI) < 30 kg/m 2 for whom a combined hormonal contraceptive is appropriate.
Limitations of Use:
ZAFEMY may be less effective in preventing pregnancy in women who weigh 198 lbs (90 kg) or more. ZAFEMY is contraindicated for use in women with BMI ≥ 30 kg/m 2 [see Contraindications (4), Warnings and Precautions (5.1) and Clinical Studies (14)].
-
2 DOSAGE AND ADMINISTRATION
To achieve maximum contraceptive effectiveness, ZAFEMY must be used exactly as directed.
Complete instructions to facilitate patient counseling on proper system usage may be found in the FDA-Approved Patient Labeling.
2.1 How to Use ZAFEMY
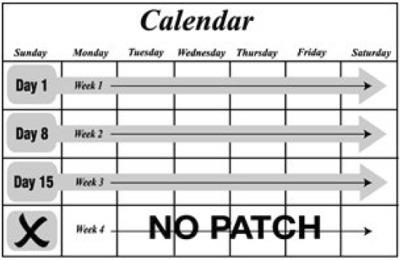
The ZAFEMY transdermal system uses a 28-day (four-week) cycle. A new patch is applied each week for three weeks (21 total days). Week Four is patch-free. Withdrawal bleeding is expected during this time.
Every new patch should be applied on the same day of the week. This day is known as the “Patch Change Day.” For example, if the first patch is applied on a Monday, all subsequent patches should be applied on a Monday. Only one patch should be worn at a time.
Do not cut, damage or alter the ZAFEMY patch in any way. If the ZAFEMY patch is cut, damaged or altered in size, contraceptive efficacy may be impaired.
On the day after Week Four ends, a new four-week cycle is started by applying a new patch. Under no circumstances should there be more than a seven-day patch-free interval between dosing cycles.
2.2 How to Start Using ZAFEMY
There are multiple options for starting the ZAFEMY patch, and the woman should choose the option that is most appropriate (see Table 1):
Table 1: Instructions for Administration
Starting ZAFEMY patch in women with no current use of hormonal contraception
The woman has two options for starting the patch and she should choose the option that is right for her:
First Day Start
- The woman should apply her first patch during the first 24 hours of her menstrual period. If a patch is applied after the first 24 hours of menstruation, non-hormonal back-up contraception (such as condoms and spermicide, or diaphragm and spermicide) is needed for the first 7 days of the first cycle only.
- The woman should apply a new patch each week for three weeks (21 total days). Every new patch should be applied on the same day of the week. This day is known as the “Patch Change Day.” For example, if the first patch is applied on a Monday, all subsequent patches should be applied on a Monday. Only one patch should be worn at a time.
- No patch is worn during Week Four (the “Patch-Free Week”). Withdrawal bleeding is expected during this time.
- On the day after Week 4 ends, a new 4-week cycle is started by applying a new patch. Under no circumstances should there be more than a 7-day patch-free interval between dosing cycles.
Sunday Start
- The woman should apply her first patch on the first Sunday after her menstrual period begins.
- With this option, a non-hormonal backup method of birth control, such as a condom and spermicide or diaphragm and spermicide, is needed for the first 7 days of the first cycle only.
- If her period starts on a Sunday, the first patch should be applied that day, and no backup contraception is needed.
Switching from another contraceptive method
Oral combination hormonal contraception (oral CHC)
- If the woman is switching from the pill to ZAFEMY patch, she should complete her current pill cycle and apply the first ZAFEMY patch on the day she would normally start her next pill
- If she does not get her period within a week after taking the last active pill, she should check with her healthcare professional to be sure that she is not pregnant, but she may go ahead and start ZAFEMY patch for contraception.
- If the patch is applied more than a week after taking the last active pill, she should use a non-hormonal back-up contraception (such as condoms and spermicide, or diaphragm and spermicide) concurrently for the first 7 days of patch use.
Transdermal system
- If the woman is switching from another contraceptive transdermal system to ZAFEMY patch, she should complete the current transdermal system cycle and apply the first ZAFEMY patch on the day the next transdermal system cycle would normally start.
- If she does not get her period within a week after removing the last transdermal system, she should check with her healthcare professional to be sure that she is not pregnant, but she may go ahead and start ZAFEMY patch for contraception. If ZAFEMY patch is applied more than a week after removal of the last patch, non-hormonal back-up contraception (such as condoms and spermicide, or diaphragm and spermicide) should be used concurrently for the first 7 days of patch use.
Vaginal ring
- If the woman is switching from the vaginal ring to ZAFEMY patch, she should complete her current vaginal ring cycle and apply the first ZAFEMY patch on the day she would normally insert her next vaginal ring.
- If she does not get her period within a week after removing the last vaginal ring, she should check with her healthcare professional to be sure that she is not pregnant, but she may go ahead and start ZAFEMY patch for contraception.
- If the patch is applied more than a week after removal of the last vaginal ring, she should use a non-hormonal back-up contraception (such as condoms and spermicide, or diaphragm and spermicide) concurrently for the first 7 days of patch use.
Injection
- If the woman is switching from an injection contraceptive to ZAFEMY patch, she should apply the first ZAFEMY patch on the day the next injection would normally occur.
Intrauterine system (IUS)
- If the woman is switching from an intrauterine system to ZAFEMY patch, she should apply the first ZAFEMY patch on the day of IUS removal. If the IUS is not removed on the first day of the menstrual cycle, non-hormonal backup contraception (such as condoms and spermicide, or diaphragm and spermicide) should be used concurrently for the first 7 days of patch use.
Implant
- If the woman is switching from an implant to ZAFEMY patch, she should apply the first ZAFEMY patch on the day of implant removal.
Progestin-only pill
- If the woman is switching from a progestin-only pill to ZAFEMY patch, she should apply the first ZAFEMY patch on the day the next progestin-only pill cycle would normally start.
Use after Childbirth
Start contraceptive therapy with ZAFEMY in women who elect not to breastfeed no sooner than 4 weeks after childbirth due to increased risk of thromboembolism. If a woman begins using ZAFEMY postpartum, and has not yet had a period, consider the possibility of ovulation and conception occurring prior to use of ZAFEMY, and instruct her to use an additional method of contraception, such as a condom and spermicide or diaphragm and spermicide, for the first seven days [see Warnings and Precautions (5.1) and Pregnancy (8.1)] .
Use after Abortion or Miscarriage
After an abortion or miscarriage that occurs in the first trimester, ZAFEMY may be started immediately. An additional method of contraception is not needed if ZAFEMY is started immediately. If use of ZAFEMY is not started within 5 days following a first trimester abortion, the woman should follow the instructions for a woman starting ZAFEMY for the first time. In the meantime she should be advised to use a non-hormonal contraceptive method. Ovulation may occur within 10 days of an abortion or miscarriage.
Start ZAFEMY no earlier than 4 weeks after a second trimester abortion or miscarriage, due to the increased risk of thromboembolic disease [see Contraindications (4) and Warnings and Precautions (5.1)] .
2.3 How to Apply ZAFEMY
CHOOSING A PLACE ON THE BODY TO PUT THE PATCH

- The patch may be placed on the upper outer arm, abdomen, buttock or back in a place where it won’t be rubbed by tight clothing. For example, it should not be placed under the waistband of clothing.
- The patch should not be placed on the breasts, on cut or irritated skin, or on the same location as the previous patch.
Before applying the patch:
- The woman should make sure the skin is clean and dry.
- She should not use lotions, creams, oils, powders, or make-up at the patch site. It may cause the patch to fail to stick properly or to become loose.
HOW TO APPLY THE PATCH

- The woman should tear open the pouch at the top edge. She should peel open the foil pouch that contains the patch and its clear plastic cover. She should gently remove the patch and its plastic cover together from the pouch, being careful not to separate the patch from the clear plastic cover.

- Using a fingernail, the woman should peel away half of the clear plastic. She should avoid touching the sticky surface with her fingers.

- The woman should apply the sticky side of the patch on the skin she has cleaned and dried. She should then remove the other half of the clear plastic and attach the entire patch to her skin.

- The woman should press firmly on the patch with the palm of her hand for 10 seconds, making sure that the whole patch adheres to her skin.
- She should run her fingers over the entire surface area to smooth out any “wrinkles” around the outer edges of the patch.
- The woman should check her patch every day to make sure all edges are sticking correctly.
WHEN TO CHANGE THE ZAFEMY PATCH
- The patch works for seven days (one week). The woman should apply a new patch on the same day each week (her Patch Change Day) for 3 weeks in a row. She must make sure she has removed her old patch prior to applying the new patch.
- During Week 4, she DOES NOT wear a patch. She must make sure she removes her old patch. (Her period should begin during this week.)
- Following Week 4, she repeats the cycle of three weekly applications followed by a patch-free week.
WHAT IF THE PATCH BECOMES LOOSE OR FALLS OFF?
The patch must stick securely to the skin to work properly. If the ZAFEMY patch becomes partially or completely detached and remains detached, insufficient drug delivery occurs. The woman should not try to reapply a patch if it is no longer sticky, if it has become stuck to itself or another surface, or if it has other material stuck to it.
If a patch edge lifts up:
- The woman should press down firmly on the patch with the palm of her hand for 10 seconds, making sure that the whole patch adheres to her skin. She should run her fingers over the entire surface area to smooth out any “wrinkles” around the edges of the patch.
- If her patch does not stick completely, she should remove it and apply a replacement patch.
- She should not tape or wrap the patch to her skin or reapply a patch that is partially adhered to clothing.
If the patch has been off or partially off:
- For less than 1 Day, she should try to reapply it. If the patch does not adhere completely, she should apply a new patch immediately. (No backup contraception is needed and her Patch Change Day will stay the same).
- For more than 1 Day or if she is not sure for how long, she may not be protected from pregnancy. To reduce this risk, she should apply a new patch and start a new 4-week cycle. She will now have a new Patch Change Day and MUST USE NON-HORMONAL BACKUP CONTRACEPTION (such as a condom and spermicide or diaphragm and spermicide) for the first week of her new cycle.
IF THE WOMAN FORGETS TO CHANGE HER PATCH
- at the start of any patch cycle (Week One/Day 1): SHE MAY NOT BE PROTECTED FROM PREGNANCY. She should apply the first patch of her new cycle as soon as she remembers. There is now a new “Patch Change Day” and a new “Day 1.” The woman must use back-up contraception, such as a condom and spermicide or diaphragm and spermicide, for the first week of the new cycle.
-
in the middle of the patch cycle (Week Two/Day 8 or Week Three/Day 15),
- for one or two days (up to 48 hours), she should apply a new patch immediately. The next patch should be applied on the usual “Patch Change Day.” No back-up contraception is needed.
- for more than two days (48 hours or more), SHE MAY NOT BE PROTECTED FROM PREGNANCY. She should stop the current contraceptive cycle and start a new four-week cycle immediately by putting on a new patch. There is now a new “Patch Change Day” and a new “Day 1.” The woman must use back-up contraception for one week.
-
at the end of the patch cycle (Week Four/Day 22),
- If the woman forgets to remove her patch, she should take it off as soon as she remembers. The next cycle should be started on the usual “Patch Change Day,” which is the day after Day 28. No back-up contraception is needed.
Under no circumstances should there be more than a seven-day patch-free interval between cycles. If there are more than seven patch-free days, THE WOMAN MAY NOT BE PROTECTED FROM PREGNANCY and back-up contraception, such as a condom and spermicide or diaphragm and spermicide, must be used for seven days. As with combined oral contraceptives, the risk of ovulation increases with each day beyond the recommended drug-free period. If she has had intercourse during such an extended patch-free interval, consider the possibility of pregnancy.
Change Day Adjustment
If the woman wishes to change her Patch Change Day, she should complete her current cycle, removing the third ZAFEMY patch on the correct day. During the patch-free week, she may select an earlier Patch Day Change by applying a new ZAFEMY patch on the desired day. In no case should there be more than 7 consecutive patch-free days.
Breakthrough Bleeding or Spotting
In the event of unscheduled or breakthrough bleeding or spotting (bleeding that occurs on the days that ZAFEMY is worn), treatment should be continued. If unscheduled bleeding persists longer than a few cycles, consider causes other than ZAFEMY.
If the woman does not have scheduled or withdrawal bleeding (bleeding that should occur during the patch-free week), she should resume treatment on the next scheduled Change Day. If ZAFEMY has been used correctly, the absence of withdrawal bleeding is not necessarily an indication of pregnancy. Nevertheless, consider the possibility of pregnancy, especially if absence of withdrawal bleeding occurs in 2 consecutive cycles. Discontinue ZAFEMY if pregnancy is confirmed.
In Case of Skin Irritation
If patch use results in uncomfortable irritation, the patch may be removed and a new patch may be applied to a different location until the next Change Day. Only one patch should be worn at a time.
Additional Instructions for Dosing
Unscheduled bleeding, spotting, and amenorrhea are frequent reasons for patients discontinuing hormonal contraceptives. In case of breakthrough bleeding, as in all cases of irregular bleeding from the vagina, consider nonfunctional causes. In case of undiagnosed persistent or recurrent abnormal bleeding from the vagina, take adequate diagnostic measures to rule out pregnancy or malignancy. If pathology has been excluded, time or a change to another method of contraception may solve the problem.
Use of Hormonal Contraceptives in the Event of a Missed Menstrual Period
- If the woman has not adhered to the prescribed schedule, consider the possibility of pregnancy at the time of the first missed period. Discontinue use of ZAFEMY if pregnancy is confirmed.
- If the woman has adhered to the prescribed regimen and misses one period, she should continue using her contraceptive patches. However, if she has adhered to the prescribed regimen, misses one period and has symptoms associated with pregnancy, rule out pregnancy. Discontinue ZAFEMY use if pregnancy is confirmed.
- If the woman has adhered to the prescribed regimen and misses two consecutive periods, rule out pregnancy. Discontinue ZAFEMY use if pregnancy is confirmed.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
ZAFEMY is contraindicated in females who are known to have or develop the following conditions:
- At high risk of arterial or venous thromboembolic events. Examples include women who:
- Smoke, if over age 35 [see Boxed Warning, and Warnings and Precautions (5.1)]
- Have deep vein thrombosis or pulmonary embolism, now or in the past [see Warnings and Precautions (5.1)]
- Have inherited or acquired hypercoagulopathies [see Warnings and Precautions (5.1)]
- Have cerebrovascular disease [see Warnings and Precautions (5.1)]
- Have coronary artery disease [see Warnings and Precautions (5.1)]
- Have thrombogenic valvular or thrombogenic rhythm diseases of the heart (for example, subacute bacterial endocarditis with valvular disease, or atrial fibrillation) [see Warnings and Precautions (5.1)]
- Have uncontrolled hypertension [see Warnings and Precautions (5.5)]
- Have diabetes mellitus with vascular disease [see Warnings and Precautions (5.7)]
- Have headaches with focal neurological symptoms or have migraine headaches with aura
- Women over age 35 with any migraine headaches [see Warnings and Precautions (5.8)]
- Body Mass Index ≥ 30 kg/m 2 [see Warnings and Precautions (5.1)]
- Liver tumors, benign or malignant, or liver disease [see Warnings and Precautions (5.3), Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)]
- Undiagnosed abnormal uterine bleeding [see Warnings and Precautions (5.9)]
- Pregnancy, because there is no reason to use hormonal contraceptives during pregnancy [see Warnings and Precautions (5.10) and Use in Specific Populations (8.1)]
- Current diagnosis of, or history of, breast cancer, which may be hormone-sensitive [see Warnings and Precautions (5.12)]
- Use of Hepatitis C drug combinations containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir, due to the potential for ALT elevations [see Warnings and Precautions (5.4)]
-
5 WARNINGS AND PRECAUTIONS
5.1 Thromboembolic Disorders and Other Vascular Conditions
- Stop ZAFEMY if an arterial or venous thromboembolic event (VTE) occurs.
- Stop ZAFEMY if there is unexplained loss of vision, proptosis, diplopia, papilledema, or retinal vascular lesions. Evaluate for retinal vein thrombosis immediately.
- If feasible, stop ZAFEMY at least 4 weeks before and through 2 weeks after major surgery or other surgeries known to have an elevated risk of VTE. Discontinue use of ZAFEMY during prolonged immobilization and resume treatment based on clinical judgment.
- Start ZAFEMY no earlier than 4 weeks after delivery, in women who are not breastfeeding. The risk of postpartum VTE decreases after the third postpartum week, whereas the risk of ovulation increases after the third postpartum week.
- Before starting ZAFEMY, evaluate any past medical history or family history of thrombotic or thromboembolic disorders and consider whether the history suggests an inherited or acquired hypercoagulopathy [see Contraindications (4)] .
Arterial Events
The use of CHCs increases the risk of cardiovascular events and cerebrovascular events, such as myocardial infarction and stroke. The risk is greater among older women (> 35 years of age), smokers, and women with hypertension, dyslipidemia, diabetes, or obesity. ZAFEMY is contraindicated in women over 35 years of age who smoke [see Contraindications (4)] . Cigarette smoking increases the risk of serious cardiovascular events from CHC use. This risk increases with age, particularly in women over 35 years of age, and with the number of cigarettes smoked.
Venous Events
The use of CHCs increases the risk of venous thromboembolic events (VTEs), such as deep vein thrombosis and pulmonary embolism. Risk factors for VTEs include smoking, obesity, and family history of VTE, in addition to other factors that contraindicate use of CHCs. The risk of VTE may be greater with ZAFEMY in women with a BMI ≥ 30 kg/m 2 compared to women with a lower BMI [see Contraindications (4)] .
While the risk of VTE associated with the use of CHCs is well-established, the rates of VTE are even greater during pregnancy, and especially during the post-partum period (see Figure 1). The frequency of VTE in women using CHCs has been estimated to be 3 to 12 cases per 10,000 woman-years.
The risk of VTE is highest during the first year of use of CHCs and when restarting hormonal contraception after a break of 4 weeks or longer. This initial higher risk declines during the first year, but users of CHCs remain at an increased risk of VTE compared to non-users of CHCs. Based on results from a few studies, there is some evidence that this is true for non-oral products as well. The risk of thromboembolic disease due to CHCs gradually disappears after CHC use is discontinued.
Figure 1 shows the risk of developing a VTE for women who are not pregnant and do not use CHCs, for women who use CHCs with a range of doses and routes of administration, for pregnant women, and for women in the post-partum period. To put the risk of developing a VTE into perspective: If 10,000 women who are not pregnant and do not use CHCs are followed for one year, between 1 and 5 of these women will develop a VTE.
Figure 1: Likelihood of Developing a VTE Within One Year Among Pregnant and Non-Pregnant Women
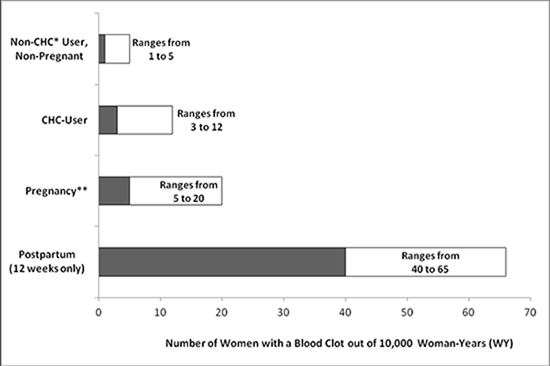
*CHC = combination hormonal contraception
**Pregnancy data based on actual duration of pregnancy in the reference studies. Based on a model assumption that pregnancy duration is nine months, the rate is 7 to 27 per 10,000 WY.
5.2 Ethinyl Estradiol Exposure
Higher estrogen exposure may increase the risk of adverse reactions, including venous thromboembolism (VTE). The Area Under the Curve (AUC) for ethinyl estradiol (EE) is approximately 60% higher in women using ZAFEMY compared to oral contraceptives containing EE 35 mcg. In contrast, the peak concentration (C max) for EE is approximately 25% lower in women using ZAFEMY [see Clinical Pharmacology (12.3)] .
5.3 Liver Disease
Impaired Liver Function
Do not use ZAFEMY in women with liver disease, such as acute viral hepatitis or severe (decompensated) cirrhosis of liver [see Contraindications (4)] . Discontinue ZAFEMY if jaundice develops. Acute or chronic disturbances of liver function may necessitate the discontinuation of CHC use until markers of liver function return to normal and CHC causation has been excluded.
Liver Tumors
ZAFEMY is contraindicated in women with benign and malignant liver tumors [see Contraindications (4)] . Hepatic adenomas are associated with CHC use. An estimate of the attributable risk is 3.3 cases/100,000 CHC users. Rupture of hepatic adenomas may cause death through intra-abdominal hemorrhage.
Studies have shown an increased risk of developing hepatocellular carcinoma in long-term (>8 years) CHC users. However, the risk of liver cancers in CHC users is less than one case per million users.
5.4 Risk of Liver Enzyme Elevations with Concomitant Hepatitis C Treatment
During clinical trials with the Hepatitis C combination drug regimen that contains ombitasvir/paritaprevir/ritonavir, with or without dasabuvir, ALT elevations greater than 5 times the upper limit of normal (ULN), including some cases greater than 20 times the ULN, were significantly more frequent in women using ethinyl estradiol-containing medications, such as CHCs. Discontinue ZAFEMY prior to starting therapy with the combination drug regimen ombitasvir/paritaprevir/ritonavir, with or without dasabuvir [see Contraindications (4)] . ZAFEMY can be restarted approximately 2 weeks following completion of treatment with the Hepatitis C combination drug regimen.
5.5 High Blood Pressure
ZAFEMY is contraindicated in women with uncontrolled hypertension or hypertension with vascular disease [see Contraindications (4)] . For women with well-controlled hypertension, monitor blood pressure and stop ZAFEMY if blood pressure rises significantly.
An increase in blood pressure has been reported in women taking hormonal contraceptives, and this increase is more likely in older women with extended duration of use. The incidence of hypertension increases with increasing concentrations of progestin.
5.6 Gallbladder Disease
Studies suggest a small increased relative risk of developing gallbladder disease among CHC users. Use of CHCs may also worsen existing gallbladder disease. A past history of CHC-related cholestasis predicts an increased risk with subsequent CHC use. Women with a history of pregnancy-related cholestasis may be at an increased risk for CHC-related cholestasis.
5.7 Carbohydrate and Lipid Metabolic Effects
Carefully monitor prediabetic and diabetic women who take ZAFEMY. CHCs may decrease glucose tolerance in a dose-related fashion. In a 6-cycle clinical trial with ZAFEMY patch there were no clinically significant changes in fasting blood glucose from baseline to end of treatment.
Consider alternative contraception for women with uncontrolled dyslipidemia. A small proportion of women will have adverse lipid changes while on hormonal contraceptives.
Women with hypertriglyceridemia, or a family history thereof, may be at an increased risk of pancreatitis when using hormonal contraceptives.
5.8 Headache
If a woman taking ZAFEMY develops new headaches that are recurrent, persistent or severe, evaluate the cause and discontinue ZAFEMY if indicated.
Consider discontinuation of ZAFEMY in the case of increased frequency or severity of migraine during hormonal contraceptive use (which may be prodromal of a cerebrovascular event).
5.9 Bleeding Irregularities
Unscheduled Bleeding and Spotting
Unscheduled (breakthrough) bleeding and spotting sometimes occur in women using ZAFEMY. Consider non-hormonal causes and take adequate diagnostic measures to rule out malignancy, other pathology, or pregnancy in the event of unscheduled bleeding, as in the case of any abnormal vaginal bleeding. If pathology and pregnancy have been excluded, time or a change to another contraceptive product may resolve the bleeding.
In the clinical trials, most women started their scheduled (withdrawal) bleeding on the fourth day of the drug-free interval, and the median duration of withdrawal bleeding was 5 to 6 days. On average, 26% of women per cycle had 7 or more total days of bleeding and/or spotting (this includes both scheduled and unscheduled bleeding and/or spotting). Three clinical studies of the efficacy of ZAFEMY in preventing pregnancy assessed scheduled and unscheduled bleeding [see Clinical Studies (14)] in 3,330 women who completed 22,155 cycles of exposure. A total of 36 (1.1%) of the women discontinued ZAFEMY at least in part, due to bleeding or spotting.
Table 2 summarizes the proportion of subjects who experienced unscheduled (breakthrough) bleeding/spotting by treatment cycle.
Table 2: Unscheduled (Breakthrough) Bleeding/Spotting (Subjects Evaluable for Efficacy)
Treatment Cycle
Pooled data from 3 studies
N=3,319n
% a
Cycle 1
2,994
18.2
Cycle 2
2,743
11.9
Cycle 3
2,699
11.6
Cycle 4
2,541
10.1
Cycle 5
2,532
9.2
Cycle 6
2,494
8.3
Cycle 7
698
8.3
Cycle 8
692
8.7
Cycle 9
654
8.6
Cycle 10
621
8.7
Cycle 11
631
8.9
Cycle 12
617
6.3
Cycle 13
611
8.0
a Percentage of subjects with breakthrough bleeding/spotting events.
Amenorrhea and Oligomenorrhea
In the event of amenorrhea, consider the possibility of pregnancy. If the patient has not adhered to the prescribed dosing schedule (missed one patch or started the patch on a day later than she should have), consider the possibility of pregnancy at the time of the first missed period and take appropriate diagnostic measures. If the patient has adhered to the prescribed regimen and misses two consecutive periods, rule out pregnancy.
Some women may encounter amenorrhea or oligomenorrhea after discontinuation of hormonal contraceptive use, especially when such a condition was pre-existent.
5.10 Hormonal Contraceptive Use Before or During Early Pregnancy
Extensive epidemiological studies have revealed no increased risk of birth defects in women who have used oral contraceptives prior to pregnancy. Studies also do not suggest a teratogenic effect, particularly in so far as cardiac anomalies and limb reduction defects are concerned, when oral contraceptives are taken inadvertently during early pregnancy. Discontinue ZAFEMY use if pregnancy is confirmed.
Administration of CHCs should not be used as a test for pregnancy [see Use in Specific Populations (8.1)] .
5.11 Depression
Carefully observe women with a history of depression and discontinue ZAFEMY if depression recurs to a serious degree.
5.12 Malignant Neoplasms
Breast Cancer
ZAFEMY is contraindicated in females who currently have or have had breast cancer because breast cancer may be hormonally sensitive [see Contraindications (4)] . Epidemiology studies have not found a consistent association between use of combined oral contraceptives (COCs) and breast cancer risk. Studies do not show an association between ever (current or past) use of COCs and risk of breast cancer. However, some studies report a small increase in the risk of breast cancer among current or recent users (<6 months since last use) and current users with longer duration of COC use [see Postmarketing Experience (6.2)] .
Cervical Cancer
Some studies suggest that combination oral contraceptive use has been associated with an increase in the risk of cervical cancer or intraepithelial neoplasia. However, there continues to be controversy about the extent to which such findings may be due to differences in sexual behavior and other factors.
5.13 Effect on Binding Globulins
The estrogen component of CHCs may raise the serum concentrations of thyroxine-binding globulin, sex hormone-binding globulin and cortisol-binding globulin. The dose of replacement thyroid hormone or cortisol therapy may need to be increased.
5.14 Monitoring
A woman who is taking hormonal contraceptive should have routine visits with her healthcare provider for a blood pressure check and for other indicated healthcare.
-
6 ADVERSE REACTIONS
The following serious adverse reactions with the use of combination hormonal contraceptives, including ZAFEMY, are discussed elsewhere in the labeling:
- Serious cardiovascular events and stroke [see Boxed Warningand Warnings and Precautions (5.1)]
- Vascular events, including venous and arterial thromboembolic events [see Warnings and Precautions (5.1)]
- Liver disease [see Warnings and Precautions (5.3)]
Adverse reactions commonly reported by users of combination hormonal contraceptives are:
- Irregular uterine bleeding
- Nausea
- Breast tenderness
- Headache
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The data described below reflect exposure to ZAFEMY in 3,330 sexually active women (3,322 of whom had safety data) who participated in three Phase 3 clinical trials designed to evaluate contraceptive efficacy and safety. These subjects received six or 13 cycles of contraception (ZAFEMY or an oral contraceptive comparator in 2 of the trials). The women ranged in age from 18 to 45 years and were predominantly white (91%).
The most common adverse reactions (≥ 5%) reported during clinical trials were breast symptoms, nausea/vomiting, headache, application site disorder, abdominal pain, dysmenorrhea, vaginal bleeding and menstrual disorders, and mood, affect and anxiety disorders. The most common events leading to discontinuation were application site reaction, breast symptoms (including breast discomfort, engorgement and pain), nausea and/or vomiting, headache and emotional lability.
Adverse drug reactions reported by ≥ 2.5% of ZAFEMY-treated subjects in these trials are shown in Table 3.
Table 3: Adverse Drug Reactions Reported by ≥ 2.5% of ZAFEMY-treated Subjects in Three Phase 3 Clinical Trials
System/Organ Class*
Adverse reactionZAFEMY
(n=3,322)Reproductive system and breast disorders
Breast symptoms †
22.4%
Dysmenorrhea
7.8%
Vaginal bleeding and menstrual disorders †
6.4%
Gastrointestinal disorders
Nausea
16.6%
Abdominal pain †
8.1%
Vomiting
5.1%
Diarrhea
4.2%
Nervous system disorders
Headache
21.0%
Dizziness
3.3%
Migraine
2.7%
General disorders and administration site conditions
Application site disorder †
17.1%
Fatigue
2.6%
Psychiatric disorders
Mood, affect and anxiety disorders †
6.3%
Skin and subcutaneous tissue disorders
Acne
2.9%
Pruritus
2.5%
Infections and infestations
Vaginal yeast infection †
3.9%
Investigations
Weight increased
2.7%
* MedDRA version 10.0
† Represents a bundle of similar terms
Additional adverse drug reactions that occurred in < 2.5% of ZAFEMY-treated subjects in the above clinical trials datasets are:
- Gastrointestinal disorders: Abdominal distension
- General disorders and administration site conditions: Fluid retention 1, malaise
- Hepatobiliary disorders: Cholecystitis
- Investigations: Blood pressure increased, lipid disorders 1
- Musculoskeletal and connective tissue disorders: Muscle spasms
- Psychiatric disorders: Insomnia, libido decreased, libido increased
- Reproductive system and breast disorders: Galactorrhea, genital discharge, premenstrual syndrome, uterine spasm, vaginal discharge, vulvovaginal dryness
- Respiratory, thoracic and mediastinal disorders: Pulmonary embolism
- Skin and subcutaneous tissue disorders: Chloasma, dermatitis contact, erythema, skin irritation
1Represents a bundle of similar terms
6.2 Postmarketing Experience
Five studies that compared breast cancer risk between ever-users (current or past use) of COCs and never-users of COCs reported no association between ever use of COCs and breast cancer risk, with effect estimates ranging from 0.90 to 1.12 (Figure 2).
Three studies compared breast cancer risk between current or recent COC users (<6 months since last use) and never users of COCs (Figure 2). One of these studies reported no association between breast cancer risk and COC use. The other two studies found an increased relative risk of 1.19 to 1.33 with current or recent use. Both of these studies found an increased risk of breast cancer with current use of longer duration, with relative risks ranging from 1.03 with less than one year of COC use to approximately 1.4 with more than 8 to 10 years of COC use.
Figure 2:
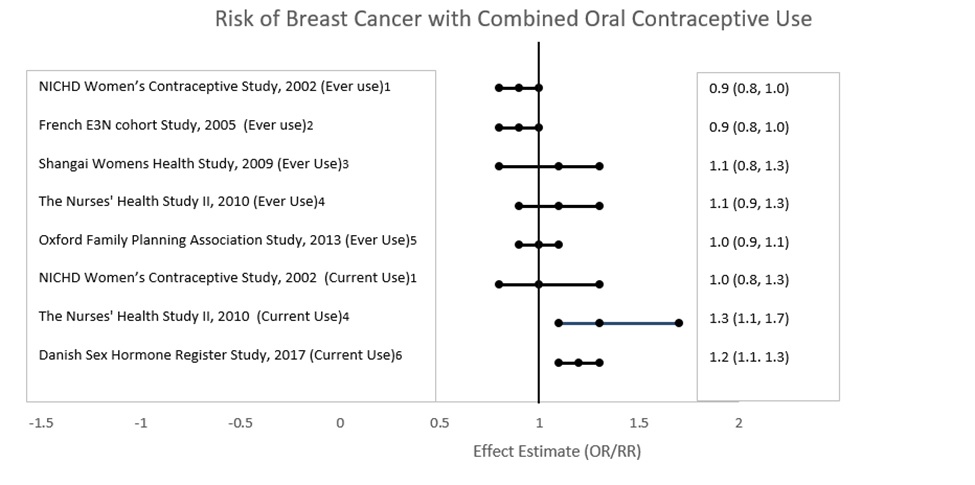
RR = relative risk; OR = odds ratio; HR = hazard ratio. “ever COC” are females with current or past COC use; “never COC use” are females that never used COCs.
The following adverse reactions (Table 4) have been identified during post-approval use of ZAFEMY. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Table 4: Alphabetical List of Adverse Drug Reactions Identified During Postmarketing Experience with ZAFEMY by System Organ Class*
System Organ Class
Adverse Drug Reactions
Cardiac disorders
Myocardial infarction †
Endocrine disorders
Hyperglycemia, insulin resistance
Eye disorders
Contact lens intolerance or complication
Gastrointestinal disorders
Colitis
General disorders and administration site conditions
Application site reaction †, edema †
Hepatobiliary disorders
Blood cholesterol abnormal, cholelithiasis, cholestasis, hepatic lesion, jaundice cholestatic, low density lipoprotein increased
Immune system disorders
Allergic reaction †, urticaria
Investigations
Blood glucose abnormal, blood glucose decreased
Metabolism and nutrition disorders
Increased appetite
Neoplasms benign, malignant and unspecified (Incl. cysts and polyps)
Breast cancer †, cervix carcinoma, hepatic adenoma, hepatic neoplasm
Nervous system disorders
Dysgeusia, migraine with aura
Psychiatric disorders
Anger, emotional disorder, frustration, irritability
Reproductive system and breast disorders
Breast mass, cervical dysplasia, fibroadenoma of breast, menstrual disorder †, suppressed lactation, uterine leiomyoma
Skin and subcutaneous tissues disorders
Alopecia, eczema, erythema multiforme, erythema nodosum, photosensitivity reaction, pruritus generalized, rash †, seborrheic dermatitis, skin reaction
Vascular disorders
Arterial thrombosis †, cerebrovascular accident †, deep vein thrombosis †, hemorrhage intracranial †, hypertension, hypertensive crisis, pulmonary embolism †, thrombosis †
* MedDRA version 10.0
† Represents a bundle of similar terms
-
7 DRUG INTERACTIONS
Consult the labeling of concurrently-used drugs to obtain further information about interactions with hormonal contraceptives or the potential for enzyme alterations.
7.1 Effects of Other Drugs on Combined Hormonal Contraceptives
Substances Decreasing the Plasma Concentrations of CHCs and Potentially Diminishing the Efficacy of CHCs:
Drugs or herbal products that induce certain enzymes, including cytochrome P450 3A4 (CYP3A4), may decrease the plasma concentrations of CHCs and potentially diminish the effectiveness of CHCs or increase breakthrough bleeding. Some drugs or herbal products that may decrease the effectiveness of hormonal contraceptives include phenytoin, barbiturates, carbamazepine, bosentan, felbamate, griseofulvin, oxcarbazepine, rifampicin, topiramate, rifabutin, rufinamide, aprepitant, and products containing St. John’s wort. Interactions between hormonal contraceptives and other drugs may lead to breakthrough bleeding and/or contraceptive failure. Counsel women to use an alternative method of contraception or a back-up method when enzyme inducers are used with CHCs, and to continue back-up contraception for 28 days after discontinuing the enzyme inducer to ensure contraceptive reliability.
Substances Increasing the Plasma Concentrations of CHCs:
Co-administration of atorvastatin or rosuvastatin and certain CHCs containing EE increase AUC values for EE by approximately 20% to 25%. Ascorbic acid and acetaminophen may increase plasma EE concentrations, possibly by inhibition of conjugation. CYP3A4 inhibitors such as itraconazole, voriconazole, fluconazole, grapefruit juice, or ketoconazole may increase plasma hormone concentrations.
Human Immunodeficiency Virus (HIV)/Hepatitis C Virus (HCV) Protease Inhibitors and Non-Nucleoside Reverse Transcriptase Inhibitors:
Significant changes (increase or decrease) in the plasma concentrations of estrogen and/or progestin have been noted in some cases of co-administration with HIV protease inhibitors (decrease [e.g., nelfinavir, ritonavir, darunavir/ritonavir, (fos)amprenavir/ritonavir, lopinavir/ritonavir, and tipranavir/ritonavir] or increase [e.g., indinavir and atazanavir/ritonavir])/HCV protease inhibitors or with non-nucleoside reverse transcriptase inhibitors (decrease [e.g., nevirapine] or increase [e.g., etravirine]).
7.2 Effects of Combined Hormonal Contraceptives on Other Drugs
CHCs containing EE may inhibit the metabolism of other compounds (e.g., cyclosporine, prednisolone, theophylline, tizanidine, and voriconazole) and increase their plasma concentrations. CHCs have been shown to decrease plasma concentrations of acetaminophen, clofibric acid, morphine, salicylic acid, and temazepam. Significant decrease in plasma concentration of lamotrigine has been shown, likely due to induction of lamotrigine glucuronidation. This may reduce seizure control; therefore, dosage adjustments of lamotrigine may be necessary.
Women on thyroid hormone replacement therapy may need increased doses of thyroid hormone because serum concentration of thyroid-binding globulin increases with use of CHCs [see Warnings and Precautions (5.13)] .
7.3 Concomitant Use with HCV Combination Therapy – Liver Enzyme Elevation
Do not co-administer ZAFEMY with HCV drug combinations containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir, due to potential for ALT elevations [see Warnings and Precautions (5.4)] .
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
There is little or no increased risk of birth defects in women who inadvertently use hormonal contraceptives during early pregnancy. Epidemiologic studies and meta-analyses have not found an increased risk of genital or non-genital birth defects (including cardiac anomalies and limb reduction defects) following exposure to low dose hormonal contraceptives prior to conception or during early pregnancy.
The administration of hormonal contraceptives to induce withdrawal bleeding should not be used as a test for pregnancy. Hormonal contraceptives should not be used during pregnancy to treat threatened or habitual abortion.
8.3 Nursing Mothers
The effects of ZAFEMY in nursing mothers have not been evaluated and are unknown. When possible, advise the nursing mother to use other forms of contraception until she has completely weaned her child. Estrogen-containing CHCs can reduce milk production in breastfeeding mothers. This is less likely to occur once breastfeeding is well-established; however, it can occur at any time in some women. Small amounts of contraceptive steroids and/or metabolites are present in breast milk.
8.4 Pediatric Use
Safety and efficacy of ZAFEMY have been established in women of reproductive age. Efficacy is expected to be the same for post-pubertal adolescents under the age of 18 and for users 18 years and older. Use of this product before menarche is not indicated.
8.5 Geriatric Use
ZAFEMY has not been studied in postmenopausal women and is not indicated in this population.
8.6 Hepatic Impairment
No studies with ZAFEMY have been conducted in women with hepatic impairment. However, steroid hormones may be poorly metabolized in patients with impaired liver function. Acute or chronic disturbances of liver function may necessitate the discontinuation of combined hormonal contraceptive use until markers of liver function return to normal and combined hormonal contraceptive causation has been excluded [see Contraindications (4) and Warnings and Precautions (5.3)] .
8.8 BMI and Weight Considerations
ZAFEMY is contraindicated in women with a BMI ≥ 30 kg/m 2 because of the potential increased risk of VTE [see Contraindications (4) and Warnings and Precautions (5.1)] .
ZAFEMY may be less effective in preventing pregnancy in women who weigh 198 lbs or more [see Clinical Studies (14)] .
- 10 OVERDOSAGE
-
11 DESCRIPTION
ZAFEMY is a transdermal system with a contact surface area of 12.5 cm 2. It contains 3.15 mg norelgestromin, USP (NGMN) and 0.289 mg ethinyl estradiol, USP (EE), and its delivery rate is approximately 150 mcg of NGMN, USP and 35 mcg of EE, USP per day. Systemic exposures (as measured by area under the curve [AUC] and steady-state concentration [C ss]) of NGMN, USP and EE, USP during use of ZAFEMY are higher and the C max is lower than those produced by an oral contraceptive containing norgestimate, USP (NGM) 250 mcg / EE, USP 35 mcg [see Boxed Warning and Clinical Pharmacology (12.3)] .
ZAFEMY is a thin, matrix-type transdermal system consisting of three layers. The backing layer is composed of a tan backing consisting of pigmented polyethylene and polyester. It provides structural support and protects the middle adhesive layer from the environment. The middle layer contains polyisobutylene/polybutene adhesive, polybutene, crospovidone, oleyl alcohol and dipropylene glycol as inactive components. The active components in this layer are the hormones, NGMN, USP and EE, USP. The third layer is the release liner, which protects the adhesive layer during storage and is removed just prior to application. It is a transparent polyethylene terephthalate (PET) film with a silicone coating on the side that is in contact with the middle adhesive layer.
The outside of the backing layer is printed with “Norelgestromin and Ethinyl Estradiol 150/35 mcg per day” in brown ink.
The structural formulas of the components are:
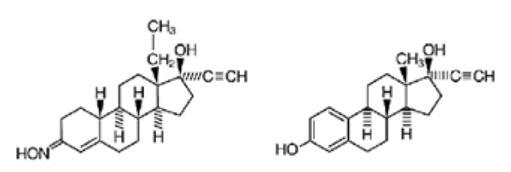
Norelgestromin, USP ethinyl estradiol, USP
Molecular weight, NGMN, USP: 327.47
Molecular weight, EE, USP: 296.41
Chemical name for NGMN, USP: 18, 19-Dinorpregn-4-en-20-yn-3-one, 13-ethyl-17-hydroxy-,3-oxime,(17α)
Chemical name for EE, USP: 19-Norpregna-1,3,5(10)-trien-20-yne-3, 17-diol,(17α)
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
NGMN is the active progestin largely responsible for the progestational activity that occurs in women following application of ZAFEMY. NGMN is also the primary active metabolite produced following oral administration of NGM, the progestin component of some oral contraceptive products.
Combination hormonal contraceptives act by suppression of gonadotropins. Although the primary mechanism of this action is inhibition of ovulation, other alterations include changes in the cervical mucus (which increase the difficulty of sperm entry into the uterus) and the endometrium (which reduce the likelihood of implantation).
12.2 Pharmacodynamics
One clinical trial assessed the return of hypothalamic-pituitary-ovarian axis function post-therapy and found that follicle-stimulating hormone (FSH), luteinizing hormone (LH), and estradiol mean values, though suppressed during therapy, returned to near baseline values during the 6 weeks post therapy.
12.3 Pharmacokinetics
Absorption
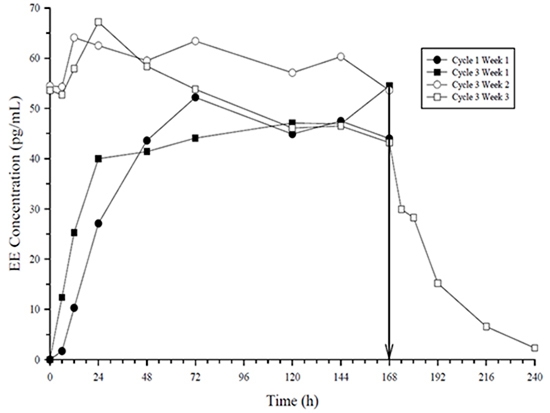
The systemic delivery rate of NGMN and EE from ZAFEMY is approximately 150 mcg of NGMN and 35 mcg of EE per day based on a comparative analysis with intravenous (IV) data. Following a single application of ZAFEMY, both NGMN and EE reach a plateau by approximately 48 hours. Pooled data from the 3 clinical studies have demonstrated that steady-state is reached within 2 weeks of application. In one of the clinical studies, C ss concentrations across all subjects ranged from 0.305 to 1.53 ng/mL for NGMN and from 23 to 137 pg/mL for EE.
Absorption of NGMN and EE following application of ZAFEMY to the buttock, upper outer arm, abdomen and upper torso (excluding breast) was examined. While absorption from the abdomen was slightly lower than from other sites, absorption from these anatomic sites was considered to be therapeutically equivalent.
The mean (%CV) PK parameters C ss and AUC 0-168 for NGMN and EE following a single buttock application of ZAFEMY are summarized in Table 5.
In multiple dose studies, AUC 0-168 for NGMN and EE was found to increase over time (Table 5). In a three-cycle study, these PK parameters reached steady-state conditions during Cycle 3 (Figures 5 and 4). Upon removal of the patch, serum levels of EE and NGMN reach very low or non-measurable levels within 3 days.
Table 5: Mean (%CV*) PK Parameters of NGMN and EE Following 3 Consecutive Cycles of ZAFEMY Wear on the Buttock
Analyte
Parameter
Cycle 1
Week 1Cycle 3
Week 1Cycle 3
Week 2Cycle 3
Week 3NGMN
C ss (ng/mL)
AUC 0-168 (ng·h/mL)
t 1/2 (h)0.70 (39.4)
107 (44.2)
nc0.70 (41.8)
105 (43.2)
nc0.80 (28.7)
132 (43.4)
nc0.70 (45.3)
120 (43.9)
32.1 (40.3)EE
C ss (pg/mL)
AUC 0-168 (pg·h/mL)
t 1/2 (h)46.4 (38.5)
6,796 (39.3)
nc47.6 (36.4)
7,160 (40.4)
nc59.0 (42.5)
10,054 (41.8)
nc49.6 (54.4)
8,840 (58.6)
21.0 (43.2)nc = not calculated, *%CV is % of Coefficient of variation = 100 (standard deviation/mean)
Figure 3: Mean Serum NGMN Concentrations (ng/mL) in Healthy Female Volunteers Following Application of ZAFEMY on the Buttock for Three Consecutive Cycles (Vertical arrow indicates time of patch removal)
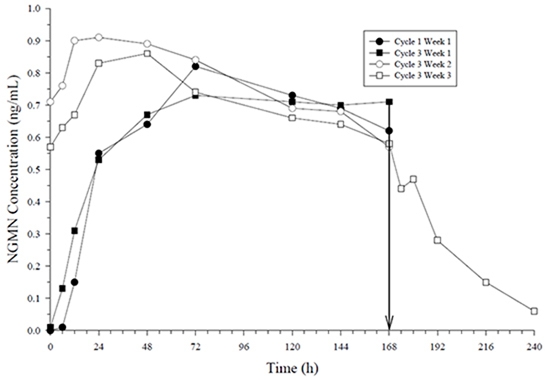
Figure 4: Mean Serum EE Concentrations (pg/mL) in Healthy Female Volunteers Following Application of ZAFEMY on the Buttock for Three Consecutive Cycles (Vertical arrow indicates time of patch removal.)
The absorption of NGMN and EE following application of ZAFEMY was studied under conditions encountered in a health club (sauna, whirlpool and treadmill) and in a cold water bath.
The results indicated that for NGMN, there were no significant treatment effects on C ss or AUC when compared to normal wear. For EE, increased exposures were observed due to sauna, whirlpool and treadmill. There was no significant effect of cold water on these parameters.
Results from a study of consecutive ZAFEMY wear for 7 days and 10 days indicated that serum concentrations of NGMN and EE dropped slightly during the first 6 hours after the patch replacement, and recovered within 12 hours. By Day 10 of patch administration, both NGMN and EE concentrations had decreased by approximately 25% when compared to Day 7 concentrations.
Metabolism
Since norelgestromin and ethinyl estradiol are delivered transdermally, first-pass metabolism (via the gastrointestinal tract and/or liver) of NGMN and EE that would be expected with oral administration does not occur. Hepatic metabolism of NGMN occurs and metabolites include norgestrel, which is highly bound to SHBG, and various hydroxylated and conjugated metabolites. EE is also metabolized to various hydroxylated products and their glucuronide and sulfate conjugates.
Distribution
NGMN and norgestrel (a serum metabolite of NGMN) are highly bound (>97%) to serum proteins. NGMN is bound to albumin and not to SHBG, while norgestrel is bound primarily to SHBG, which limits its biological activity. EE is extensively bound to serum albumin and induces an increase in the serum concentrations of SHBG (see Table 5).
Elimination
Following removal of patches, the elimination kinetics of NGMN and EE were consistent for all studies with half-life values of approximately 28 hours and 17 hours, respectively. The metabolites of NGMN and EE are eliminated by renal and fecal pathways.
Transdermal versus Oral Contraceptives
The ZAFEMY transdermal patch delivers EE and NGMN over a seven-day period while oral contraceptives (containing NGM 250 mcg / EE 35 mcg) are administered on a daily basis. Figures 5 and 6 present mean PK profiles for EE and NGMN following administration of an oral contraceptive (containing NGM 250 mcg / EE 35 mcg) compared to the 7-day transdermal ZAFEMY patch (containing NGMN 3.15 mg / EE 0.289 mg) during Cycle 2 in 32 healthy female volunteers.
Figure 5: Mean Serum Concentration-Time Profiles of NGMN Following Once-Daily Administration of an Oral Contraceptive for 2 Cycles or Application of ZAFEMY for 2 Cycles to the Buttock in Healthy Female Volunteers. [Oral contraceptive: Cycle 2, Days 15 to 21, ZAFEMY: Cycle 2, Week 3]
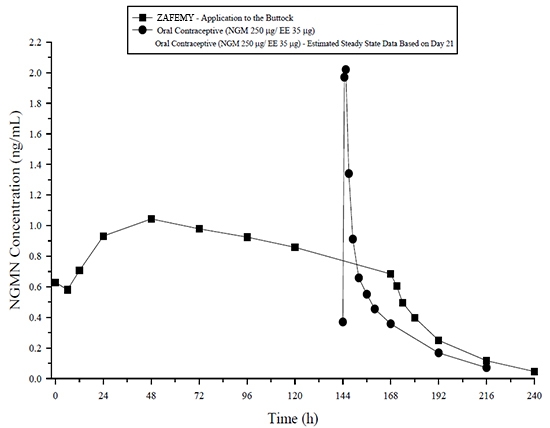
Figure 6: Mean Serum Concentration-Time Profiles of EE Following Once-Daily Administration of an Oral Contraceptive for 2 Cycles or Application of ZAFEMY for 2 Cycles to the Buttock in Healthy Female Volunteers. [Oral contraceptive: Cycle 2, Days 15 to 21, ZAFEMY: Cycle 2, Week 3]
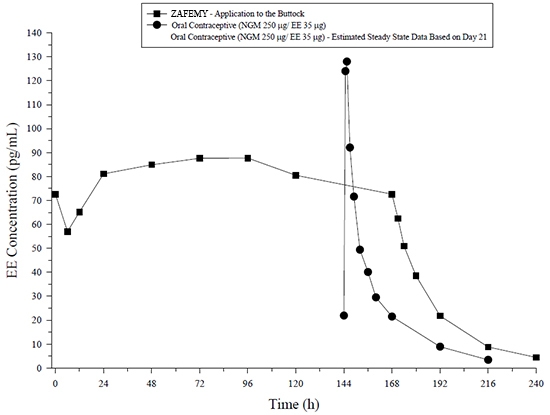
Table 6 provides the mean (%CV) for NGMN and EE pharmacokinetic (PK) parameters.
Table 6: Mean (%CV) NGMN and EE Steady-State Pharmacokinetic Parameters Following Application of ZAFEMY and Once-Daily Administration of an Oral Contraceptive (containing NGM 250 mcg / EE 35 mcg) in Healthy Female Volunteers
Parameter
ZAFEMY*
ORAL CONTRACEPTIVE †
NGMN ‡
C max (ng/mL)
1.12 (33.6)
2.16 (25.2)
AUC 0-168 (ng·h/mL)
145 (36.8)
123 (30.2) §
C ss (ng/mL)
0.888 (36.6)
0.732 (30.2) ¶
EE
C max (pg/mL)
97.4 (31.6)
133 (27.7)
AUC 0-168 (pg·h/mL)
12,971 (33.1)
8,281 (26.9) §
C ss (pg/mL)
80.0 (33.5)
49.3 (26.9) ¶
* Cycle 2, Week 3
† Cycle 2, Day 21
‡ NGM is rapidly metabolized to NGMN following oral administration
§ Average weekly exposure, calculated as AUC 24 × 7
¶ C avg
In general, overall exposure for NGMN and EE (AUC and C ss) was higher in subjects treated with ZAFEMY for both Cycle 1 and Cycle 2, compared to that for the oral contraceptive, while C max values were higher in subjects administered the oral contraceptive. Under steady-state conditions, AUC 0-168 and C ss for EE were approximately 55% and 60% higher, respectively, for the transdermal patch, and the C max was about 35% higher for the oral contraceptive, respectively. Inter-subject variability (%CV) for the PK parameters following delivery from ZAFEMY was higher relative to the variability determined from the oral contraceptive. The mean PK profiles are different between the two products and caution should be exercised when making a direct comparison of these PK parameters.
In Table 7, percent change in concentrations (%CV) of markers of systemic estrogenic activity (Sex Hormone Binding Globulin [SHBG] and Corticosteroid Binding Globulin [CBG]) from Cycle 1 Day 1 to Cycle 1 Day 22 is presented. Percent change in SHBG concentrations was higher for ZAFEMY users compared to women taking the oral contraceptive; percent change in CBG concentrations was similar for ZAFEMY and oral contraceptive users. Within each group, the absolute values for SHBG were similar for Cycle 1, Day 22 and Cycle 2, Day 22.
Table 7: Mean Percent Change (%CV) in SHBG and CBG Concentrations Following Once-Daily Administration of an Oral Contraceptive (containing NGM 250 mcg / EE 35 mcg) for One Cycle and Application of ZAFEMY for One Cycle in Healthy Female Volunteers Parameter ZAFEMY
(% change from Day 1 to Day 22)ORAL CONTRACEPTIVE
(% change from Day 1 to Day 22)SHBG 334 (39.3) 200 (43.2) CBG 153 (40.2) 157 (33.4) Drug Interactions
In a PK drug interaction study, oral administration of tetracycline HCl, 500 mg four times daily for 3 days prior to and 7 days during wear of ZAFEMY did not significantly affect the PK of NGMN or EE.
Use in Specific Populations
Effects of Age, Body Weight, Body Surface Area and Race
The effects of age, body weight, body surface area and race on the PK of NGMN and EE were evaluated in 230 healthy women from nine pharmacokinetic studies of single 7-day applications of ZAFEMY. For both NGMN and EE, increasing age, body weight and body surface area each were associated with slight decreases in C ss and AUC values. However, only a small fraction (10% to 25%) of the overall variability in the PK of NGMN and EE following application of ZAFEMY may be associated with any or all of the above demographic parameters. There was no significant effect of race with respect to Caucasians, Hispanics and Blacks.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, and Impairment of Fertility
See Warnings and Precautions (5.3, 5.12) and Use in Specific Populations (8.1).
Norelgestromin was tested in in vitro mutagenicity assays (bacterial plate incorporation mutation assay, CHO/HGPRT mutation assay, chromosomal aberration assay using cultured human peripheral lymphocytes) and in one in vivo test (rat micronucleus assay) and found to have no genotoxic potential.
-
14 CLINICAL STUDIES
In 3 large clinical trials lasting 12 months, in North America, Europe and South Africa, 3,330 women (ages 18 to 45) completed 22,155 cycles of ZAFEMY patch use, the pregnancy rate in women aged 18 to 35 years was 1.07 (95% confidence interval 0.60, 1.76) per 100 woman-years of ZAFEMY patch use. The racial distribution was 91% Caucasian, 4.9% Black, 1.6% Asian, and 2.4% Other.
With respect to weight, 5 of the 15 pregnancies reported with ZAFEMY patch use were among women with a baseline body weight ≥ 198 lbs., which constituted < 3% of the study population. The greater proportion of pregnancies among women at or above 198 lbs. was statistically significant and suggests that ZAFEMY patch may be less effective in these women.
Patch Adhesion
In the clinical trials with ZAFEMY patch, approximately 2% of the cumulative number of patches completely detached and 3% partially detached. The proportion of subjects with at least 1 patch that completely detached ranged from 2% to 6%, with a reduction from Cycle 1 (6%) to Cycle 13 (2%). For instructions on how to manage detachment of patches, refer to Dosage and Administration (2).
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
ZAFEMY (norelgestromin and ethinyl estradiol transdermal system) is available in one strength of 150 mcg/day NGMN, USP and 35 mcg/day EE, USP.
ZAFEMY is a 12.5 cm 2 system with rounded corners with tan backing printed with “Norelgestromin and Ethinyl Estradiol 150/35 mcg per day” in brown ink, protected with a removable translucent oversized dimple slit-release liner. Each patch contains 3.15 mg of norelgestromin, USP and 0.289 mg of ethinyl estradiol, USP.
Each transdermal system is packaged in a protective pouch.
ZAFEMY is available in folding cartons of 1 cycle each (NDC: 42291-930-03); each cycle contains 3 systems.
16.2 Special Precautions for Storage and Disposal
Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Store patches in their protective pouches. Apply immediately upon removal from the protective pouch.
Do not store in the refrigerator or freezer.
Used patches still contain some active hormones. The sticky sides of the patch should be folded together and the folded patch placed in a sturdy container, preferably with a child-resistant cap, and the container thrown in the trash. Used patches should not be flushed down the toilet.
For more information, go to www.avkare.com or call 1-855-361-3993
-
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling ( Patient Information and Instructions for Use)
17.1 General
Counsel patients about the following information:
- Cigarette smoking increases the risk of serious cardiovascular events from combined hormonal contraceptive use, and that women who are over 35 years old and smoke should not use combined hormonal contraceptives.
- The use of CHCs increases the risk of VTE. However, pregnancy increases the risk of VTE as much or more than the use of CHCs. The risk of VTE in women using CHCs is 3 to 12 cases per 10,000 woman-years. The risk of VTE is highest during the first year of use of CHCs and when restarting hormonal contraception after a break of 4 weeks or longer. The risk of thromboembolic disease due to CHCs gradually disappears after use is discontinued.
- ZAFEMY does not protect against HIV infection (AIDS) and other sexually transmitted infections.
- The Warnings and Precautions associated with combined hormonal contraceptives.
- ZAFEMY is not to be used during pregnancy; if pregnancy occurs during use of ZAFEMY, instruct the patient to stop further use.
- Apply a single patch the same day every week (Weeks 1 through 3). Instruct patients what to do in the event a patch is missed. See “WHAT IF I FORGET TO CHANGE MY PATCH?” section in FDA-Approved Patient Labeling.
- Use a back-up or alternative method of contraception when enzyme inducers are used with ZAFEMY.
- Combined hormonal contraceptives may reduce breast milk production; this is less likely to occur if breastfeeding is well established.
- Women who start combined hormonal contraceptives postpartum, and who have not yet had a period, should use an additional method of contraception until they have used a patch for 7 consecutive days.
- Amenorrhea may occur. Consider pregnancy in the event of amenorrhea. Rule out pregnancy in the event of amenorrhea in two or more consecutive cycles, amenorrhea in one cycle if the woman has not adhered to the dosing schedule, or if associated with symptoms of pregnancy, such as morning sickness or unusual breast tenderness.
- If the ZAFEMY patch becomes partially or completely detached and remains detached, insufficient drug delivery occurs.
- A patch should not be re-applied if it is no longer sticky, becomes stuck to itself or another surface, has other material stuck to it, or has become loose or fallen off before. If a patch cannot be re-applied, a new patch should be applied immediately. Supplemental adhesives or wraps should not be used.
- A woman may not be protected from pregnancy if a patch is partially or completely detached for ≥24 hours (or if the woman is not sure how long the patch has been detached). She should start a new cycle immediately by applying a new patch. Back-up contraception, such as a condom and spermicide or diaphragm and spermicide, must be used for the first week of the new cycle.
All trademarks are the property of their respective owners.
Manufactured for:
AvKARE
Pulaski, TN 38478
Manufactured by:
Amneal Pharmaceuticals LLC
Piscataway, NJ 08854
Mfg. Rev. 04-2022-04 AV 04/23
-
Patient Information
ZAFEMY® (za feʹ my)
(norelgestromin and ethinyl estradiol transdermal system)What is the most important information I should know about ZAFEMY?
Do not use ZAFEMY if you smoke cigarettes and are over 35 years old. Smoking increases your risk of serious cardiovascular side effects from hormonal birth control methods, including death from heart attack, blood clots or stroke. This risk increases with age and the number of cigarettes you smoke.
Do not use ZAFEMY if you have an increased risk for blood clots.
Do not use ZAFEMY if your Body Mass Index (BMI) is 30 kg/m 2 or more. Women with a BMI of 30 kg/m 2 or more who use ZAFEMY may be at a higher risk for developing blood clots compared to women with a BMI lower than 30 kg/m 2.
Hormonal birth control methods help to lower the chances of becoming pregnant. They do not protect against HIV infection (AIDS) and other sexually transmitted infections. What is ZAFEMY?
ZAFEMY is a birth control patch for women with a BMI less than 30 kg/m 2. It contains two female hormones, an estrogen called ethinyl estradiol, and a progestin called norelgestromin.
Hormones from ZAFEMY get into the blood stream and are processed by the body differently than hormones from birth control pills. You will be exposed to about 60% more estrogen if you use ZAFEMY than if you use a typical birth control pill containing 35 micrograms of estrogen. In general, increased estrogen may increase the risk of side effects.
How well does ZAFEMY work?
Your chance of getting pregnant depends on how well you follow the directions for using ZAFEMY. The better you follow the directions, the less chance you have of getting pregnant.
In clinical studies, 1 to 2 out of 100 women got pregnant during the first year that they used ZAFEMY.
ZAFEMY may not be as effective in women weighing more than 198 lbs. (90 kg). If you weigh more than 198 lbs. (90 kg), talk to your healthcare provider about which method of birth control is right for you.
The following chart shows the chance of getting pregnant for women who use different methods of birth control. Each box on the chart contains a list of birth control methods that are similar in effectiveness. The most effective methods are at the top of the chart. The box on the bottom of the chart shows the chance of getting pregnant for women who do not use birth control and are trying to get pregnant.
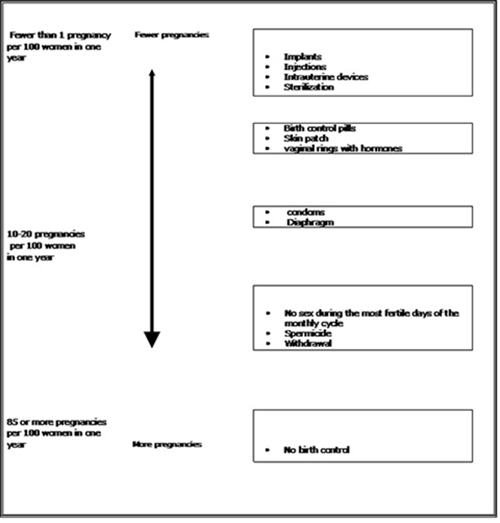
Do not use ZAFEMY if you:
- smoke and are over 35 years old
- have or have had blood clots in your arms, legs, eyes or lungs
- have an inherited problem that makes your blood clot more than normal
- have had a stroke
- have had a heart attack
- have certain heart valve problems or heart rhythm problems that can cause blood clots to form in the heart
- have high blood pressure that medicine cannot control
- have diabetes with kidney, eye, nerve, or blood vessel damage
- have had certain kinds of severe migraine headaches with aura, numbness, weakness or changes in vision, or have any migraine headaches if you are over age 35
- have a BMI of 30 or more
- have liver disease, including liver tumors, take any Hepatitis C drug combination containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir. This may increase levels of the liver enzyme “alanine aminotransferase” (ALT) in the blood.
- have unexplained vaginal bleeding
- are pregnant or think you may be pregnant. However, ZAFEMY is not known to cause birth defects when used by accident during pregnancy.
- have had breast cancer or any cancer that is sensitive to female hormones
Hormonal birth control methods may not be a good choice for you if you have ever had jaundice (yellowing of the skin or eyes) caused by pregnancy or related to previous use of hormonal birth control.
Tell your healthcare provider if you have ever had any of the above conditions. Your healthcare provider may recommend another method of birth control.
Before you use ZAFEMY, tell your healthcare provider:
- about all your medical conditions
- if you are pregnant or think you are pregnant
- if you are scheduled for surgery. ZAFEMY may increase your risk of blood clots after surgery. You should stop using your ZAFEMY patch at least 4 weeks before you have surgery and not restart it until at least 2 weeks after your surgery.
- if you are scheduled for any laboratory tests. Certain blood tests may be affected by hormonal birth control methods.
- are breastfeeding or plan to breastfeed. Hormonal birth control methods that contain estrogen, like ZAFEMY, may decrease the amount of milk you make. A small amount of hormones from the ZAFEMY patch may pass into your breast milk. Consider another method of birth control until you are ready to stop breastfeeding.
Tell your healthcare provider about all medicines and herbal products that you take.
Some medicines and herbal products may make hormonal birth control less effective, including, but not limited to:
- certain seizure medicines (carbamazepine, felbamate, oxcarbazepine, phenytoin, rufinamide, and topiramate)
- aprepitant
- barbiturates
- bosentan
- griseofulvin
- certain combinations of HIV medicines (nelfinavir, ritonavir, ritonavir-boosted protease inhibitors)
- certain non-nucleoside reverse transcriptase inhibitors (nevirapine)
- rifampin and rifabutin
- St. John’s wort
Use another birth control method (such as a condom and spermicide or diaphragm and spermicide) when you take medicines that may make the ZAFEMY patch less effective.
Some medicines and grapefruit juice may increase your level of the hormone ethinyl estradiol if used together, including:
- acetaminophen
- ascorbic acid
- medicines that affect how your liver breaks down other medicines (itraconazole, ketoconazole, voriconazole, and fluconazole)
- certain HIV medicines (atazanavir, indinavir)
- atorvastatin
- rosuvastatin
- etravirine
Hormonal birth control methods may interact with lamotrigine, an anti-seizure medicine used for epilepsy. This may increase the risk of seizures, so your healthcare provider may need to adjust the dose of lamotrigine.
Women on thyroid replacement therapy may need increased doses of thyroid hormone.
Know the medicines you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine.
How should I use ZAFEMY?
- For detailed instructions, see the step-by-step instructions for using ZAFEMY at the end of this Patient Information.
- Use ZAFEMY exactly as your healthcare provider tells you to use it.
- Wear 1 ZAFEMY patch at a time. Make sure you remove your old ZAFEMY patch before applying your new ZAFEMY patch.
- Do not skip using any ZAFEMY patches, even if you do not have sex often.
- ZAFEMY is applied in a 4-week cycle.
- Apply your ZAFEMY patch 1 time each week for 3 weeks (21 total days).
- Apply each new ZAFEMY patch on the same day of the week. This day will be your "Patch Change Day." For example, if you apply your first ZAFEMY patch on a Monday, all of your ZAFEMY patches should be applied on a Monday.
- Do not apply your ZAFEMY patch during Week 4. Make sure you remove your old ZAFEMY patch. This is your patch-free week. Your menstrual period should start during your patch-free week.
- Begin a new 4 week cycle by applying a new ZAFEMY patch on the day after Week 4 ends. Repeat the cycle of 3 weekly applications followed by a patch-free week.
- Your ZAFEMY patch should never be off for more than 7 days in a row. If your ZAFEMY patch is off for more than 7 days in a row and you have sex during this time, you could become pregnant.
- If you miss a period you might be pregnant. Some women miss their periods or have light periods on hormonal birth control methods even when they are not pregnant. Call your healthcare provider if you miss 1 period and have not used your ZAFEMY patch every day or you miss 2 periods in a row.
What are the possible side effects of ZAFEMY?
See "What is the most important information I should know about ZAFEMY?"
ZAFEMY may cause serious side effects, including:
- blood clots. Like pregnancy, hormonal birth control methods increase the risk of serious blood clots (see following graph), especially in women who have other risk factors, such as smoking, high blood pressure, high levels of fat in the blood, diabetes, obesity, a family history of blood clots, or age greater than 35. This increased risk is highest when you first start using hormonal birth control and when you restart the same or different hormonal birth control after not using it for a month or more. Some studies have reported that women who use ZAFEMY have a higher risk of getting a blood clot. Talk with your healthcare provider about your risk of getting a blood clot before using ZAFEMY or deciding which type of birth control is right for you.
It is possible to die or be permanently disabled from a problem caused by a blood clot, such as a heart attack or a stroke. Some examples of serious blood clots are blood clots in the:
- legs (deep vein thrombosis)
- lungs (pulmonary embolus)
- eyes (loss of eyesight)
- heart (heart attack)
- brain (stroke)
To put the risk of developing a blood clot into perspective: If 10,000 women who are not pregnant and do not use hormonal birth control are followed for one year, between 1 and 5 of these women will develop a blood clot. The figure below shows the likelihood of developing a serious blood clot for women who are not pregnant and do not use hormonal birth control, for women who use hormonal birth control, for pregnant women, and for women in the first 12 weeks after delivering a baby.
Likelihood of Developing a Serious Blood Clot (Venous Thromboembolism [VTE])
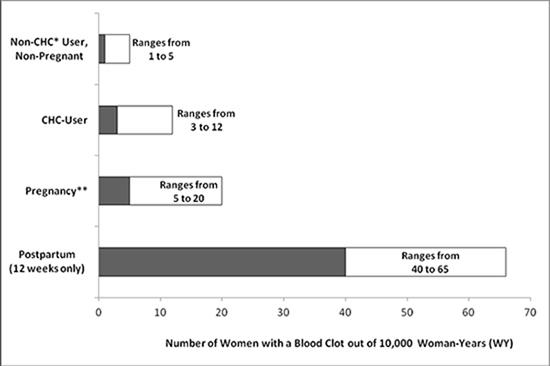
*CHC=combination hormonal contraception
**Pregnancy data based on actual duration of pregnancy in the reference studies. Based on a model assumption that pregnancy duration is nine months, the rate is 7 to 27 per 10,000 WY.
Call your healthcare provider right away if you have:
- leg pain that will not go away
- sudden shortness of breath
- sudden blindness, partial or complete
- severe pain or pressure in your chest
- sudden, severe headache unlike your usual headaches
- weakness or numbness in an arm or leg, or trouble speaking
- yellowing of the skin or eyeballs
Other serious risks include
- liver problems including liver tumors
- gallbladder disease
- high blood pressure
The most common side effects of ZAFEMY are:
- breast symptoms (discomfort, swelling, or pain)
- nausea
- headache
- skin irritation, redness, pain, swelling, itching or rash at the patch application site
- stomach pain
- pain during menstruation
- vaginal bleeding and menstrual disorders, such as spotting or bleeding between periods
- mood, affect and anxiety disorders
Some women have spotting or light bleeding, breast tenderness, or feel sick to their stomach during ZAFEMY use. If these symptoms occur, do not stop using the ZAFEMY patch. The problem will usually go away. If it doesn't go away, check with your healthcare provider.
Less common side effects are:
- acne
- less sexual desire
- bloating or fluid retention
- blotchy darkening of your skin, especially your face
- high blood sugar, especially in women with diabetes
- high fat (cholesterol, triglycerides) levels in the blood
- depression, especially if you have had depression in the past. Call your healthcare provider immediately if you have any thoughts of harming yourself.
- problems tolerating contact lenses
- weight gain
Tell your healthcare provider about any side effect that bothers you or that does not go away.
These are not all the possible side effects of ZAFEMY. For more information, ask your healthcare provider or pharmacist.
Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store and throw away used ZAFEMY patches?
- Store at room temperature between 59°F to 86°F (15°C to 30°C).
- Do not store ZAFEMY patches outside of their pouches. Apply immediately upon removal from the protective pouch.
- Do not store in the refrigerator or freezer.
- Used ZAFEMY patches still contain some active hormones. To throw away the ZAFEMY patch, fold the sticky side of the patch together, place it in a sturdy child-proof container, and place this container in the trash. Do not flush used ZAFEMY patches down the toilet.
- Return unused, unneeded, or expired patches to your pharmacist.
Keep ZAFEMY and all medicines out of the reach of children.
General information about the safe and effective use of ZAFEMY
Medicines are sometimes prescribed for purposes other than those listed in Patient Information. Do not use ZAFEMY for a condition for which it was not prescribed. Do not give ZAFEMY to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your pharmacist or healthcare provider for information about ZAFEMY that is written for health professionals.
For more information, go to www.avkare.com or call 1-855-361-3993What are the ingredients in ZAFEMY?
Active ingredient: norelgestromin, USP and ethinyl estradiol, USP
Inactive ingredient: pigmented polyethylene and polyester backing, polyisobutylene/polybutene adhesive, polybutene, crospovidone, oleyl alcohol, dipropylene glycol, polyethylene terephthalate (PET) film with a silicone coating.Does hormonal birth control cause cancer?
It is not known if hormonal birth control causes breast cancer. Some studies, but not all, suggest that there could be a slight increase in the risk of breast cancer among current users with longer duration of use.
If you have breast cancer now, or have had it in the past, do not use hormonal birth control because some breast cancers are sensitive to hormones.
Women who use hormonal birth control methods may have a slightly higher chance of getting cervical cancer. However, this may be due to other reasons such as having more sexual partners.
What should I know about my period when using ZAFEMY?
When you use ZAFEMY you may have bleeding and spotting between periods, called unplanned bleeding. Unplanned bleeding may vary from slight staining between menstrual periods to breakthrough bleeding which is a flow much like a regular period. Unplanned bleeding occurs most often during the first few months of ZAFEMY use, but may also occur after you have been using the patch for some time. Such bleeding may be temporary and usually does not indicate any serious problems. It is important to continue using the patch on schedule. If the unplanned bleeding or spotting is heavy or lasts for more than a few days, you should discuss this with your healthcare provider.
What if I miss my scheduled period when using ZAFEMY?Some women miss periods on hormonal birth control, even when they are not pregnant. However, if you go 2 or more months in a row without a period, or you miss your period after a month where you did not use all of your patches correctly, or you have symptoms associated with pregnancy, such as morning sickness or unusual breast tenderness, call your healthcare provider because you may be pregnant. Stop taking ZAFEMY if you are pregnant.
What if I want to become pregnant?You may stop using ZAFEMY whenever you wish. Consider a visit with your healthcare provider for a pre-pregnancy checkup before you stop using the patch.
Zafemy is a registered trademark of Amneal Pharmaceuticals LLC.
Manufactured for:
AvKARE
Pulaski, TN 38478
Manufactured by:
Amneal Pharmaceuticals LLC
Piscataway, NJ 08854
Mfg. Rev. 04-2022-04 AV 04;23
-
INSTRUCTIONS FOR USE
ZAFEMY ® (za feʹ my)
(norelgestromin and ethinyl estradiol transdermal system)
ZAFEMY is for skin use only.
Do not cut, damage, or alter the ZAFEMY patch in any way.
How to start using your ZAFEMY patch:

Figure A
- If you are not currently using hormonal birth control, you have 2 ways to begin using your ZAFEMY patch. Choose the way that is best for you:
- First day start: Apply your first ZAFEMY patch during the first 24 hours of your menstrual period.
- Sunday start: Apply your first ZAFEMY patch on the first Sunday after your menstrual period begins. Use a non-hormonal contraceptive method of birth control, such as a condom and spermicide or diaphragm and spermicide, for the first 7 days of your first cycle only. If your period starts on Sunday, apply your first ZAFEMY patch that day, and no back-up birth control is needed.
- If you are changing from oral hormone birth control pills, a vaginal contraceptive ring or another contraceptive transdermal patch to the ZAFEMY patch:
- Complete your current oral hormone birth control pill cycle, vaginal ring cycle or contraceptive transdermal patch cycle. Apply your first ZAFEMY patch on the day you would normally start your next oral birth control pill, patch or insert your next vaginal ring.
- If you do not get your period within 1 week after taking your last active pill, removing your last vaginal ring or contraceptive transdermal patch, check with your healthcare provider to make sure you are not pregnant. You may still go ahead and start ZAFEMY patch for contraception.
- If you apply your ZAFEMY patch more than 1 week after taking your last active oral hormone birth control pill, removing your last vaginal ring or contraceptive transdermal patch, use a non-hormonal contraceptive method, such as a condom and spermicide or diaphragm and spermicide, with the ZAFEMY patch for the first 7 days of patch use.
- If you are starting ZAFEMY after childbirth:
- If you are not breastfeeding, wait 4 weeks before using ZAFEMY and use a non-hormonal contraceptive method of birth control, such as a condom and spermicide or diaphragm and spermicide, for the first 7 days of your first cycle only. If you have had sex since your baby was born, wait for your first period, or see your healthcare provider to make sure you are not pregnant before starting ZAFEMY.
- If you are starting ZAFEMY after a miscarriage or abortion:
- You may start ZAFEMY immediately after a miscarriage or abortion that occurs in the first 12 weeks (first trimester) of pregnancy. You do not need to use another contraceptive method.
- If you do not start ZAFEMY within 5 days after a first trimester miscarriage or abortion, use a non-hormonal contraceptive method of birth control, such as a condom and spermicide or diaphragm and spermicide, while you wait for your period to start. You have 2 ways to begin using your ZAFEMY patch. Choose the way that is best for you:
- First day start: Apply your first ZAFEMY patch during the first 24 hours of your menstrual period.
- Sunday start: Apply your first ZAFEMY patch on the first Sunday after your menstrual period begins. Use a non-hormonal contraceptive method of birth control, such as a condom and spermicide or diaphragm and spermicide, for the first 7 days of your first cycle only. If your period starts on Sunday, apply your first ZAFEMY patch that day, and no back-up birth control is needed.
- If you are starting ZAFEMY after a miscarriage or abortion that occurs after the first 12 weeks of pregnancy (second trimester), wait 4 weeks before using ZAFEMY and use a non-hormonal contraceptive method of birth control, such as a condom and spermicide or diaphragm and spermicide, for the first 7 days of your first cycle only. If you have had sex since your miscarriage or abortion, wait for your first period, or see your healthcare provider to make sure you are not pregnant before starting ZAFEMY.
Figure B is a picture of the ZAFEMY patch.

Figure B
Step 1. Choose a place on your body for your ZAFEMY patch

- The ZAFEMY patch may be placed on your upper outer arm, abdomen, buttock or back in a place where it will not be rubbed by tight clothing. Avoid the waistline because clothing and belts may cause your patch to be rubbed off.
- Do not apply the patch to your breasts.
- Apply the ZAFEMY patch only to skin that is clean, dry, and free of any powder, make-up, cream, oil, or lotion.
- Do not apply the ZAFEMY patch to cut or irritated skin, or in the same location as the previous ZAFEMY patch.
Step 2: Apply your ZAFEMY patch

- Tear open the pouch at the top edge. Peel open the foil pouch that contains the ZAFEMY patch and its clear plastic cover. Gently remove the ZAFEMY patch and its plastic cover together from the pouch, being careful not to separate the patch from the clear plastic cover.

- Using a fingernail, peel away half of the clear plastic. Avoid touching the sticky surface with your fingers.

- Apply the sticky side of the ZAFEMY patch to clean, dry skin. Remove the other half of the clear plastic and apply the entire patch to your skin.

- Press firmly on the ZAFEMY patch with the palm of your hand for 10 seconds, making sure that the whole patch sticks to your skin.
- Run your fingers over the entire surface area to smooth out any “wrinkles” around the outer edges of the ZAFEMY patch.
- Check your ZAFEMY patch every day to make sure all edges are sticking correctly.
Step 3: Throwing away your ZAFEMY patch
- To throw away the ZAFEMY patch, fold the sticky side of the patch together, place it in a sturdy child-proof container, and place the container in the trash.
- Used ZAFEMY patches should not be flushed in the toilet.
Important notes:
- Your ZAFEMY patch must stick securely to your skin to work properly.
- Do not try to reapply a ZAFEMY patch if it is no longer sticky, if it has become stuck to itself or another surface, or if it has other material stuck to it. Do not tape or wrap the patch to your skin or reapply a patch that is partially adhered to clothing.
- If your ZAFEMY patch edge lifts up:
- Press down firmly on the patch with the palm of your hand for 10 seconds, making sure that the whole patch sticks to your skin. Run your fingers over the entire surface area to smooth out any “wrinkles” around the edges of the ZAFEMY patch.
- If your ZAFEMY patch does not stick completely, remove it and apply a replacement ZAFEMY patch.
- Do not tape or wrap the ZAFEMY patch to your skin or reapply a ZAFEMY patch that is partially stuck to clothing.
- If your ZAFEMY patch has been off or partially off:
- For less than 1 Day, try to reapply it. If the ZAFEMY patch does not stick completely, apply a new ZAFEMY patch immediately. No back-up contraception is needed and your "Patch Change Day" will stay the same.
- For more than 1 Day or if you are not sure for how long, you could become pregnant. To reduce this chance, apply a new ZAFEMY patch and start a new 4 week cycle. You will now have a new "Patch Change Day." Use a non-hormonal back-up contraception method such as a condom and spermicide or diaphragm and spermicide for the first week of your new 4 week ZAFEMY cycle.
- Talk to your healthcare provider about a replacement ZAFEMY patch prescription so you will always have an extra ZAFEMY patch available if needed.
- If you want to move your "Patch Change Day" to a different day of the week, finish your current cycle. Remove your third ZAFEMY patch on the correct day.
- During week 4, the "Patch Free Week" (Day 22 through Day 28), you may choose an earlier "Patch Change Day" by applying a new patch on the day you prefer. You now have a new Day 1 and a new "Patch Change Day."
- If your ZAFEMY patch becomes uncomfortable or your application site is red, painful or swollen, change your ZAFEMY patch. Remove your ZAFEMY patch and apply a new patch to a new location until your next "Patch Change Day."
- If you forget to change or remove your ZAFEMY patch:
- At the start of any patch cycle (Week 1, Day 1):
- You could become pregnant. You must use a back-up contraception method for 7 days. Apply the first ZAFEMY patch of your new cycle as soon as you remember. You now have a new "Patch Change Day" and a new Day 1.
- In the middle of your patch cycle (Week 2 or Week 3):
- If you forget to change your ZAFEMY patch for 1 or 2 days, apply a new ZAFEMY patch as soon as you remember. Apply your next patch on your normal "Patch Change Day." No back-up contraception method is needed.
- If you forget to change your ZAFEMY patch for more than 2 days, you could become pregnant. Start a new 4 week cycle as soon as you remember by putting on a new ZAFEMY patch. You now have a different "Patch Change Day" and a new Day 1. You must use a back-up contraception method for the first 7 days of your new cycle.
- At the end of your patch cycle (Week 4):
- If you forget to remove your ZAFEMY patch, take it off as soon as you remember. Start your next cycle on your normal "Patch Change Day," the day after Day 28. No back-up contraception method is needed.
- If you forget to apply your ZAFEMY patch at the start of your next patch cycle, you could become pregnant. Apply the first ZAFEMY patch of your new cycle as soon as you remember. You now have a new "Patch Change Day" and a new Day 1. Use a non-hormonal back-up contraception method such as a condom and spermicide or diaphragm and spermicide for the first 7 days of your new 4 week ZAFEMY cycle.
- If you have trouble remembering to change your ZAFEMY patch, talk to your healthcare provider about how to make patch changing easier or about using another method of contraception.
- If you are not sure how to use your ZAFEMY patch:
- Use a back-up contraception method such as a condom and spermicide or diaphragm and spermicide anytime you have sex. Make sure to have 1 of these non-hormonal contraception methods ready at all times.
- Talk to your healthcare provider for instructions on using your ZAFEMY patch.
This Patient Information and Instructions for Use have been approved by the U.S. Food and Drug Administration.
Zafemy is a registered trademark of Amneal Pharmaceuticals LLC
Manufactured for:
AvKAREPulaski, TN 38478
Manufactured by:
Amneal Pharmaceuticals LLC
Piscataway, NJ 08854
Mfg. Rev. 04-2022-04 AV 04/23
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ZAFEMY
norelgestromin and ethinyl estradiol patchProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 42291-930 Route of Administration TRANSDERMAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NORELGESTROMIN (UNII: R0TAY3X631) (NORELGESTROMIN - UNII:R0TAY3X631) NORELGESTROMIN 150 ug in 1 d ETHINYL ESTRADIOL (UNII: 423D2T571U) (ETHINYL ESTRADIOL - UNII:423D2T571U) ETHINYL ESTRADIOL 35 ug in 1 d Inactive Ingredients Ingredient Name Strength POLYBUTENE (1400 MW) (UNII: 1NA5AO9GH7) CROSPOVIDONE (UNII: 2S7830E561) OLEYL ALCOHOL (UNII: 172F2WN8DV) DIPROPYLENE GLYCOL (UNII: E107L85C40) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 42291-930-03 3 in 1 CARTON 05/15/2023 1 NDC: 42291-930-01 1 in 1 POUCH 1 7 d in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA213950 05/15/2023 Labeler - AvKARE (796560394)
Trademark Results [ZAFEMY]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ZAFEMY 88769372 not registered Live/Pending |
Amneal Pharmaceuticals LLC 2020-01-22 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.