SODIUM CHLORIDE injection
Sodium Chloride by
Drug Labeling and Warnings
Sodium Chloride by is a Prescription medication manufactured, distributed, or labeled by Baxter Healthcare Corporation, Baxter Healthcare S.A.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
0.9% Sodium Chloride Injection, USP in the MINI-BAG Plus Container is a sterile, nonpyrogenic solution for intravenous administration after admixture with a single dose powdered or liquid (up to 10 mL) drug vial. It contains no antimicrobial agents. The nominal pH is 5.0 (4.5 to 7.0). Each 100 mL contains 900 mg of Sodium Chloride, USP (NaCl). The osmolarity is 308 mOsmol/L (calculated). It contains 154 mEq/L sodium and 154 mEq/L chloride.
The MINI-BAG Plus Container is a standard diluent container with an integral drug vial adaptor. It allows for drug admixture after connection to a single dose powdered or liquid (up to 10 mL) drug vial having a 20 mm closure. A breakaway seal in the tube between the vial adaptor and the container is broken to allow transfer of the diluent into the vial and reconstitution of the drug. The MiniBag Plus product mechanically prohibits the transfer of contaminants into and out of the system during and after docking, minimizing environmental and personal exposure. The reconstituted drug is then transferred from the vial into the container diluent and mixed to result in an admixture for delivery to the patient.
The VIAFLEX Plastic Container is fabricated from a specially formulated polyvinyl chloride (PL 146 Plastic). Exposure to temperatures above 25°C/77°F during transport and storage will lead to minor losses in moisture content. Higher temperatures lead to greater losses. It is unlikely that these minor losses will lead to clinically significant changes within the expiration period. The amount of water that can permeate from inside the container into the overwrap is insufficient to affect the solution significantly. Solutions in contact with the plastic container can leach out certain chemical components from the plastic in very small amounts. However, biological testing was supportive of the safety of the plastic container materials.
- CLINICAL PHARMACOLOGY
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
Hypersensitivity
Hypersensitivity and infusion reactions, including hypotension, pyrexia, tremor, chills, urticaria, rash, and pruritus have been reported with 0.9% Sodium Chloride Injection, USP.
Stop the infusion immediately if signs or symptoms of a hypersensitivity reaction develop, such as tachycardia, chest pain, dyspnea and flushing. Appropriate therapeutic countermeasures must be instituted as clinically indicated.
Electrolyte Imbalances
Fluid Overload
Depending on the volume and rate of infusion, and the patient’s underlying clinical condition, the intravenous administration of Sodium Chloride Injection, USP can cause fluid distrurbance such as overhydration/hypervolemia and congested states, including pulmonary congestion and edema.
Avoid 0.9% Sodium Chloride Injection, USP in patients with or at risk for fluid and/or solute overloading. If use cannot be avoided, fluid balance, electrolyte concentrations, and acid base balance, as needed and especially during prolonged use.
Hyponatremia
0.9% Sodium Chloride Injection, USP may cause hyponatremia. Hyponatremia can lead to acute hyponatremic encephalopathy characterized by headache, nausea, seizures, lethargy, and vomiting. Patients with brain edema are at particular risk of severe, irreversible and life-threatening brain injury.
The risk of hospital-acquired hyponatremia is increased in patients with cardiac or pulmonary failure, and in patients with non-osmotic vasopressin release (including SIADH) treated with high volume of Sodium Chloride Injection, USP.
The risk for hyponatremia is increased in pediatric patients, elderly patients, postoperative patients, in those with psychogenic polydipsia, and in patients treated with medications that increase the risk of hyponatremia (such as diuretics, certain antiepileptic and psychotropic medications). See DRUG INTERACTIONS.
Patients at increased risk for developing complications of hyponatremia such as hyponatremic encephalopathy, include pediatric patients , women (in particular pre-menopausal women), patients with hypoxemia, and patients with underlying central nervous system disease. Avoid 0.9% Sodium Chloride Injection, USP in patients with or at risk for hyponatremia. If use cannot be avoided, monitor serum sodium concentrations.
Rapid correction of hyponatremia is potentially dangerous with risk of serious neurologic complications. Brain adaptations reducing risk of cerebral edema make the brain vulnerable to injury when chronic hyponatremia is too rapidly corrected, which is known as osmotic demyelination syndrome (ODS). To avoid complications, monitor serum sodium and chloride concentrations, fluid status, acid-base balance, and signs of neurologic complications.
Hypernatremia
Hypernatremia may occur with Sodium Chloride Injection, USP. Conditions that may increase the risk of hypernatremia, fluid overload and edema (central and peripheral), include patients with: primary hyperaldosteronism; secondary hyperaldosteronism associated with, for example, hypertension, congestive heart failure, liver disease (including cirrhosis), renal disease (including renal artery stenosis, nephrosclerosis); and pre-eclampsia.
Certain medications, such as corticosteroids or corticotropin, may also increase risk of sodium and fluid retention, see DRUG INTERACTIONS.
Avoid 0.9% Sodium Chloride Injection, USP in patients with, or at risk for, hypernatremia. If use cannot be avoided, monitor serum sodium concentrations.
Rapid correction of hypernatremia is potentially dangerous with risk of serious neurologic complications. Excessively rapid correction of hypernatremia is also associated with a risk for serious neurologic complications such as osmotic demyelination syndrome (ODS) with risk of seizures and cerebral edema.
For use only with a single dose powdered or liquid (up to 10 mL) drug vial with a 20 mm closure.
Do not administer unless drug is completely dissolved and drug vial is empty. Do not remove drug vial at any time prior to or during administration.
-
PRECAUTIONS
Patients with Severe Renal Impairment
Administration of 0.9%Sodium Chloride Injection, USP in patients with or at risk of severe renal impairment, may result in hypernatremia and/or fluid overload (see WARNINGS). Avoid Sodium Chloride Injection, USP in patients with severe renal impairment or conditions that may cause sodium and/or potassium retention, fluid overload, or edema. If use cannot be avoided, monitor patients with severe renal impairment for development of these adverse reactions.
Drug Interactions
Other Products that Affect Fluid and/or Electrolyte Balance
Administration of Sodium Chloride Injection, USP to patients treated concomitantly with drugs associated with sodium and fluid retention,- may increase the risk of hypernatremia and volume overload. Avoid use of Sodium Chloride Injection, USP in patients receiving such products, such as corticosteroids or corticotropin. If use cannot be avoided, monitor serum electrolytes, fluid balance and acid-base balance.
Lithium
Renal sodium and lithium clearance may be decreased during during administration of Sodium Chloride Injection, USP. Monitor serum may result in increased lithium concentrations during concomitant use.
Renal sodium and lithium clearance may be increased during administration of Sodium Chloride Injection, USP. Monitor serum lithium concentrations during concomitant use.
Other Drugs that Increase the Risk of Hyponatremia
Administration of 0.9% Sodium Chloride Injection, USP in patients treated concomitantly with medications associated with hyponatremia may increase the risk of developing hyponatremia.
Avoid use of 0.9% Sodium Chloride Injection, USP in patients receiving products, such as diuretics, and certain antiepileptic and psychotropic medications. Drugs that increase the vasopressin effect reduce renal electrolyte free water excretion and may also increase the risk of hyponatremia following treatment with intravenous fluids. If use cannot be avoided, monitor serum sodium concentrations.
Pregnancy
There are no adequate and well controlled studies with Sodium Chloride Injection, USP, in pregnant women and animal reproduction studies have not been conducted with this drug. Therefore, it is not known whether Sodium Chloride Injection, USP can cause fetal harm when administered to a pregnant woman. Sodium Chloride Injection, USP should be given during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
It is not known whether this drug is present in human milk. Because many drugs are present in human milk, caution should be exercised when Sodium Chloride Injection, USP is administered to a nursing woman.
Pediatric Use
The use of Sodium Chloride Injection, USP in pediatric patients is based on clinical practice. (See DOSAGE AND ADMINISTRATION.)
Closely monitor plasma electrolyte concentrations in pediatric patients who may have impaired ability to regulate fluids and electrolytes. In very low birth weight infants, excessive or rapid administration of Sodium Chloride Injection, USP may result in increased serum osmolality and risk of intracerebral hemorrhage.
Children (including neonates and older children) are at increased risk of developing hyponatremia as well as for developing hyponatremic encephalopathy.
Geriatric Use
Geriatric patients are at increased risk of developing electrolyte imbalances. Sodium Chloride Injection, USP is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function.
Therefore, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. Consider monitoring renal function in elderly patients.
-
ADVERSE REACTIONS
Post-Marketing Adverse Reactions
The following adverse reactions have been identified during postapproval use of 0.9% Sodium Chloride Injection, USP. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The following adverse reactions have been reported in the post-marketing experience during use of 0.9% Sodium Chloride Injection, USP and include the following:
Hypersensitivity reactions: hypersensitivity/infusion reactions, including hypotension, pyrexia, tremor, chills, urticaria, rash, and pruritus.
General disorders and administration site conditions: infusion site reactions, such as infusion site erythema, injection site streaking, burning sensation, and infusion site urticaria.
Metabolism and nutrition disorders: hypernatremia, hyperchloremic metabolic acidosis, and hyponatremia, which may be symptomatic.
Nervous System Disorders: Hyponatremic encephalopathy.
If an adverse reaction does occur, discontinue the infusion, evaluate the patient, institute appropriate therapeutic countermeasures and save the remainder of the fluid for examination if deemed necessary.
-
OVERDOSAGE
Excessive administration of:
- 0.9% Sodium Chloride Injection, USP can cause hypernatremia.
- 0.9% Sodium Chloride Injection, USP can cause fluid overload (which can lead to pulmonary and/or peripheral edema). See WARNINGS and ADVERSE REACTIONS.
When assessing an overdose, any additives in the solution must also be considered. The effects of an overdose may require immediate medical attention and treatment.
Interventions include discontinuation of Sodium Chloride Injection, USP administration, dose reduction, and other measures as indicated for the specific clinical constellation (e.g., monitoring of fluid balance, electrolyte concentrations and acid base balance).
-
DOSAGE AND ADMINISTRATION
Important Administration Instructions
- 0.9 % Sodium Chloride Injection, USP is intended for intravenous administration using sterile equipment.
- Do not connect flexible plastic containers in series in order to avoid air embolism due to possible residual air contained in the primary container.
- Set the vent to the closed position on a vented intravenous administration set to prevent air embolism.
- Use a dedicated line without any connections to avoid air embolism.
- Do not pressurize intravenous solutions contained in flexible plastic containers to increase flow rates in order to avoid air embolism due to incomplete evacuation of residual air in the container.
- Prior to infusion, visually inspect the solution for particulate matter and discoloration. The solution should be clear and there should be no precipitates. Do not administer unless solution is clear, and container is undamaged.
Dosing Information
The choice of product, dosage, volume, rate, and duration of administration is dependent upon the age, weight and clinical condition of the patient and concomitant therapy, and administration should be determined by a physician experienced in intravenous fluid therapy.
Introduction of Additives
Additives may be incompatible.
Evaluate all additions to the plastic container for compatibility and stability of the resulting preparation. Consult with a pharmacist, if available.
If, in the informed judgment of the physician, it is deemed advisable to introduce additives, use aseptic technique. Mix thoroughly when additives have been introduced. After addition, if there is a discoloration and/or the appearance of precipitates, insoluble complexes or crystals, do not use. Do not store solutions containing additives. Discard any unused portion.
-
HOW SUPPLIED
0.9% Sodium Chloride Injection, USP in MINI-BAG Plus Container is available as follows:
Code
Size (mL)
NDC
2B0042
50
0338-0553-11
2B0043
100
0338-0553-18
Exposure of pharmaceutical products to heat should be minimized. Avoid excessive heat. It is recommended the product be stored at room temperature (25°C/77°F).
-
DIRECTIONS FOR USE
For Information on Risk of Air Embolism – see DOSAGE AND ADMINISTRATION.
To Open
Tear overwrap down side at slit and remove solution container. Visually inspect the container. If the outlet port protector is damaged, detached, or not present, discard container as solution path sterility may be impaired. Some opacity of the plastic due to moisture absorption during the sterilization process may be observed. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually.
Prior to use, check that the vial adaptor cover is intact. Check the solution container for minute leaks by squeezing inner bag firmly. If leaks are found or if the vial adaptor cover is not intact, discard product as sterility may be impaired.
To Assemble and Reconstitute
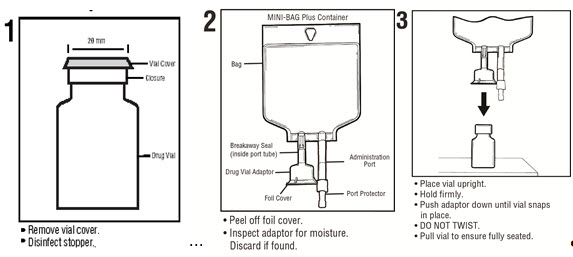
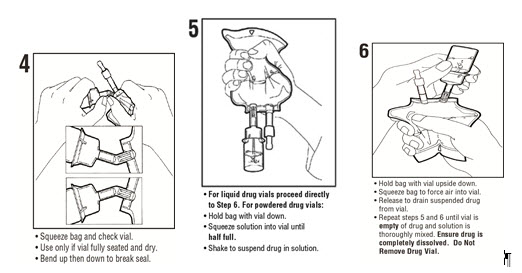
See diagram for detailed instructions.
MINI-BAG Plus Container Directions
Only For Single Dose Powdered or Liquid (up to 10 mL)
Drug Vials with 20 mm Closures
Use Aseptic TechniqueAssembly
Reconstitution
- 7. Remove port protector. Attach administration set per its directions.
- 8.
Hang container on I.V. pole and prime set per directions. Ensure that vial is empty of drug and solution. Repeat step 6 if drug and solution remain in vial.
Warning: Do not use in series connections. - 9. Administer medication per directions. Use within specified time for drug stability.
- SPL UNCLASSIFIED SECTION
-
PACKAGE LABEL - PRINCIPLE DISPLAY PANEL
LOT EXP
0.9% Sodium
Chloride Injection USP2B0042
NDC: 0338-0553-11MINI-BAG Plus Container
50mL EACH 50 mL CONTAINS 450 mg SODIUM CHLORIDE
USP pH 5.0 (4.5 TO 7.0) mEq/50 mL SODIUM 8
CHLORIDE 8 OSMOLARITY 308 mOsmol/L (CALC) STERILE
NONPYROGENIC READ PACKAGE INSERT FOR FULL INFORMATION
ADDITIVES MAY BE INCOMPATIBLE DOSAGE INTRAVENOUSLY AS
DIRECTED BY A PHYSICIAN CAUTIONS MUST NOT BE USED IN SERIES
CONNECTIONS DO NOT USE UNLESS SOLUTION IS CLEAR
RX ONLYBaxter Logo
BAXTER HEALTHCARE CORPORATION
DEERFIELD IL 60015 USA
MADE IN USAVIAFLEX SINGLE DOSE CONTAINER
PL 146 PLASTIC
BAXTER VIAFLEX MINI-BAG
AND PL 146 ARE TRADEMARKS OF
BAXTER INTERNATIONAL INCBREAK SEAL AND MIX BEFORE USE
Carton Label
Lot:PXX Exp: XX XX
QTY: 80-50 mL 2B0042
NDC: 0338-0553-11
0.9% SODIUM CHLORIDE INJECTION, USP
IN MINI-BAG PLUS CONTAINER
(17) XXXX00 (10) PXX
(01) 50303380553117
-
INGREDIENTS AND APPEARANCE
SODIUM CHLORIDE
sodium chloride injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0338-0553 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 900 mg in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0338-0553-11 50 mL in 1 BAG; Type 0: Not a Combination Product 12/07/1992 2 NDC: 0338-0553-18 100 mL in 1 BAG; Type 0: Not a Combination Product 12/07/1992 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020178 12/07/1992 Labeler - Baxter Healthcare Corporation (005083209) Establishment Name Address ID/FEI Business Operations Baxter Healthcare Corporation 189326168 ANALYSIS(0338-0553) , MANUFACTURE(0338-0553) , LABEL(0338-0553) , PACK(0338-0553) , STERILIZE(0338-0553) Establishment Name Address ID/FEI Business Operations Baxter Healthcare Corporation 091171389 ANALYSIS(0338-0553) , LABEL(0338-0553) , MANUFACTURE(0338-0553) , PACK(0338-0553) , STERILIZE(0338-0553) Establishment Name Address ID/FEI Business Operations Baxter Healthcare Corporation 194684502 ANALYSIS(0338-0553) Establishment Name Address ID/FEI Business Operations Baxter Healthcare Corporation 059140764 ANALYSIS(0338-0553) Establishment Name Address ID/FEI Business Operations Baxter Healthcare S.A. 988899845 ANALYSIS(0338-0553)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.