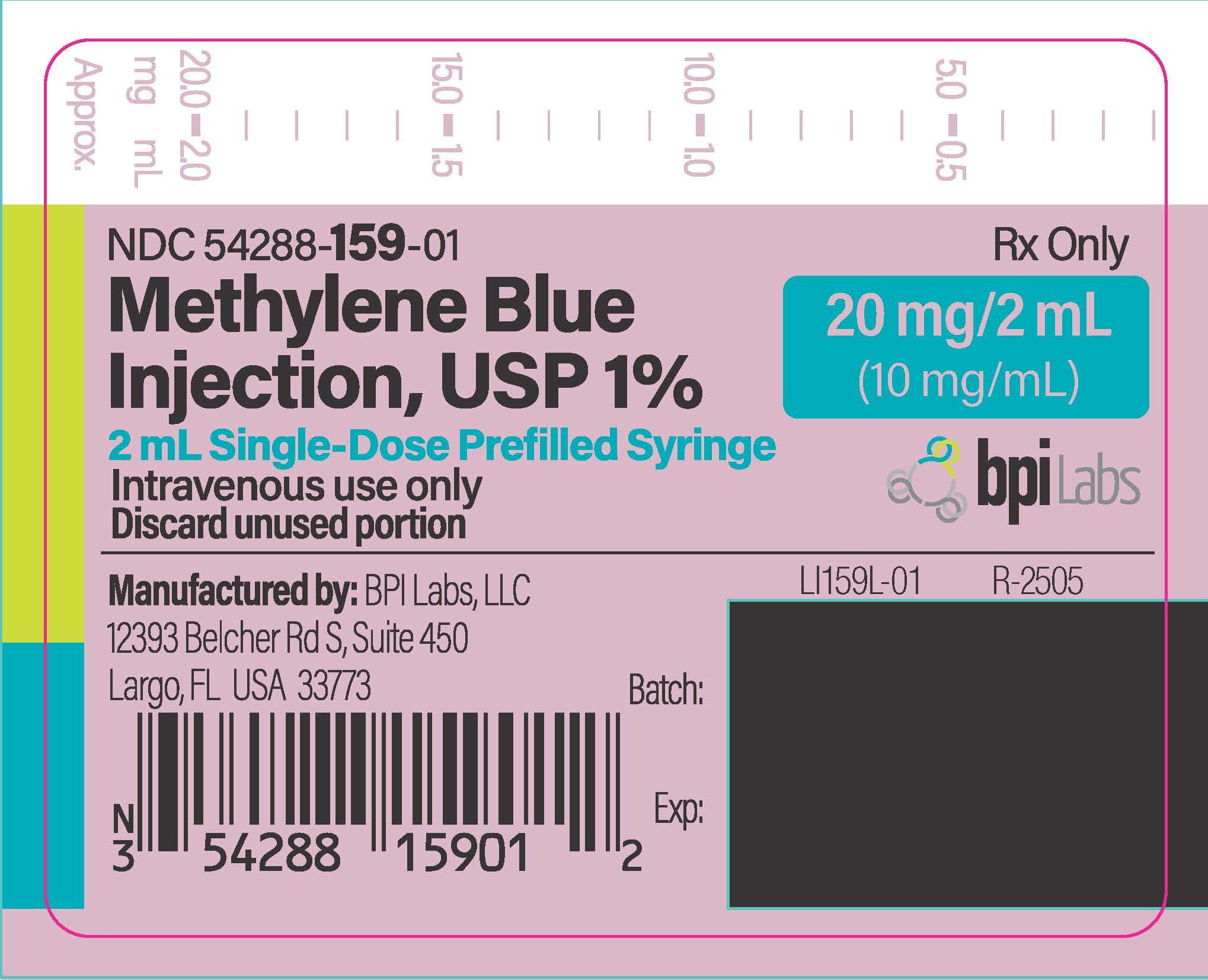
METHYLENE BLUE injection
Methylene Blue by
Drug Labeling and Warnings
Methylene Blue by is a Prescription medication manufactured, distributed, or labeled by BPI LABS LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
BOXED WARNING
(What is this?)
WARNING: SEROTONIN SYNDROME WITH CONCOMITANT USE OF SEROTONERGIC DRUGS
Methylene Blue Injection may cause serious or fatal serotonergic syndrome when used in combination with serotonergic drugs. Avoid concomitant use of Methylene Blue Injection with selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), and monoamine oxidase inhibitors (see WARNINGS and PRECAUTIONS, Drug Interactions).
-
DESCRIPTION :
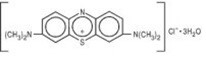
Methylene Blue Injection is a sterile solution of Phenothiazin-5-ium, 3, 7-bis (dimethylamino)-, chloride, trihydrate. Each mL contains methylene blue, 10 mg in water for injection q.s. pH adjusted with sodium hydroxide and/or hydrochloric acid when necessary.
The structural formula is:
The molecular formula is:
C16H18ClN3S3H2O MW = 373.90 -
CLINICAL PHARMACOLOGY :
Methylene blue will produce two opposite actions on hemoglobin. Low concentrations will convert methemoglobin to hemoglobin. High concentrations convert the ferrous iron of reduced hemoglobin to ferric iron which results in the formation of methemoglobin.
Methylene blue is metabolized in the body to leukomethylene blue which is excreted primarily in the urine. Some unchanged drug is also excreted in the urine. (1)
- INDICATIONS AND USAGE :
-
CONTRAINDICATIONS :
Methylene blue can cause fetal harm when administered to a pregnant woman. An association exists between the use of methylene blue in amniocentesis and atresia of the ileum and jejunum, ileal occlusions, and other adverse effects in the neonate. (2, 3)
Methylene blue is contraindicated in women who are or may become pregnant. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus.
Intraspinal and subcutaneous injections are contraindicated.
Methylene blue is contraindicated in patients with a known hypersensitivity to the drug.
-
WARNINGS :
Methylene blue should not be given by subcutaneous or intrathecal injection.
Methylene blue is a potent monoamine oxidase inhibitor:Methylene blue has been demonstrated to be a potent monoamine oxidase inhibitor (MAOI) and may cause potentially fatal serotonin toxicity (serotonin syndrome) when combined with serotonin reuptake inhibitors (SRIs). (4) (see DRUG INTERACTIONS.) Serotonin toxicity is characterized by development of neuromuscular hyperactivity (tremor, clonus, myoclonus, and hyperreflexia, and, in the advanced stage, pyramidal rigidity); autonomic hyperactivity (diaphoresis, fever, tachycardia, tachypnoea, and mydriasis); and altered mental status (agitation, excitement, and in the advanced stage, confusion). If methylene blue is judged to be indicated, SRIs must be ceased, prior to treatment/procedure/surgery.
-
PRECAUTIONS :
Drug Interactions:Methylene blue may interact with any drug that acts as a serotonin reuptake inhibitor (SRI) including, amongst others, selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), norepinephrine-dopamine reuptake inhibitors (NDRIs), triptans and ergot alkaloids; such combinations may have the consequence of potentially fatal serotonin toxicity (serotonin syndrome). Methylene blue should not be co- administered with any drug that acts as an SRI.
Pregnancy: Pregnancy Category X:Epidemiologic evidence exists that methylene blue is a teratogen. An association exists between the use of methylene blue in amniocentesis and atresia of the ileum and jejunum, ileal occlusions, and other adverse effects in the neonate. (2,3) Methylene blue injection should not be administered to pregnant women during amniocentesis due to the risk of teratogenicity and other newborn adverse effects (see CONTRAINDICATIONS).
Glucose-6-Phosphate Dehydrogenase Deficiency (G6PD Deficiency):Methylene blue should be avoided in patients with G6PD deficiency due to the risk of paradoxical methemoglobinemia and hemolysis. (5,6)
Renal Failure:Methylene blue should be used with caution in patients with severe renal impairment (see CLINICAL PHARMACOLOGY).
Methylene blue must be injected intravenously very slowly over a period of several minutes to prevent local high concentration of the compound from producing additional methemoglobin. Do not exceed recommended dosage.
Large intravenous doses of methylene blue produce nausea, abdominal and precordial pain, dizziness, headache, profuse sweating, mental confusion, and the formation of methemoglobin.
-
DOSAGE AND ADMINISTRATION :
0.1 to 0.2 mL per kg body weight (0.045 to 0.09 mL per pound body weight). Inject methylene blue intravenously very slowly over a period of several minutes.
Methylene blue must be injected intravenously very slowly over a period of several minutes to prevent local high concentration of the compound from producing additional methemoglobin. Do not exceed recommended dosage. Parenteral drug products should be inspected visually for particulate matter and discoloration, whenever solution and container permit.
- HOW SUPPLIED :
- STORAGE :
-
REFERENCES :
- DiSanto AR, Wagner JG. Pharmacokinetics of highly ionized drugs II: methylene blue – absorption, metabolism, and excretion in man and dog after oral administration. J Pharm Sci. 1972; 61:1086- 1090.
- Cragan JD. Teratogen update: methylene blue. Teratology. 1999; 60:42-48.
- Kidd SA, Lancaster PA, Anderson JC, Boogert A, Fisher CC, Robertson R, et al. Fetal death after exposure to methylene blue dye during mid-trimester amniocentesis in twin pregnancy. Prenat Diagn. 1996; 16:39-47.
- Ramsay RR, Dunford C, Gillman PK. Methylene blue and serotonin toxicity: inhibition of monoamine oxidase A (MAOA) confirms a theoretical prediction. Br J Pharmacol. 2007; 152:946-51.
- Beutler E. G6PD Deficiency. Blood. 1994; 84:3613-3636.
- Youngster I, Arcavi L, Schechmaster R, Akayzen Y, Popliski H, Shimonov J, Beig S, Berkovitch M. Medications and glucose-6-phosphate dehydrogenase deficiency: an evidence-based review. Drug Saf. 2010; 33:713-726.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
METHYLENE BLUE
methylene blue injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 54288-159 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHYLENE BLUE (UNII: T42P99266K) (METHYLENE BLUE CATION - UNII:ZMZ79891ZH) METHYLENE BLUE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 54288-159-05 5 in 1 PACKAGE 05/15/2023 1 NDC: 54288-159-01 2 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 05/15/2023 Labeler - BPI LABS LLC (078627620) Establishment Name Address ID/FEI Business Operations BPI LABS LLC 078627620 analysis(54288-159) , manufacture(54288-159) , label(54288-159)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.