GIAZO- balsalazide disodium tablet, film coated
GIAZO by
Drug Labeling and Warnings
GIAZO by is a Prescription medication manufactured, distributed, or labeled by Salix Pharmaceuticals, Inc, Nexgen Pharma, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use GIAZO safely and effectively. See full prescribing information for GIAZO.
GIAZO® (balsalazide disodium) tablets, for oral use
Initial U.S. Approval: 2000INDICATIONS AND USAGE
GIAZO is a locally acting aminosalicylate indicated for the treatment of mildly to moderately active ulcerative colitis in male patients 18 years of age and older. (1)
Limitations of Use
DOSAGE AND ADMINISTRATION
Three 1.1 g GIAZO tablets 2 times a day (6.6 g/day) with or without food for up to 8 weeks. (2)
DOSAGE FORMS AND STRENGTHS
Tablets: 1.1 g balsalazide disodium (3)
CONTRAINDICATIONS
Patients with hypersensitivity to salicylates or to any of the components of GIAZO tablets or balsalazide metabolites (4)
WARNINGS AND PRECAUTIONS
- Exacerbation of the symptoms of ulcerative colitis was reported. Observe patients closely for worsening of these symptoms while on treatment. (5.1)
- Renal impairment may occur. Assess renal function at the beginning of treatment and periodically during treatment. (5.2)
- Use with caution with pre-existing liver disease. (5.3)
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥2%) in male UC patients are anemia, diarrhea, pharyngolaryngeal pain, and urinary tract infection. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Salix Pharmaceuticals, Inc. at 1-800-321-4576 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 1/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Exacerbations of Ulcerative Colitis
5.2 Renal Impairment
5.3 Use in Hepatic Impairment
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Ulcerative Colitis
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
GIAZO is indicated for the treatment of mildly to moderately active ulcerative colitis in male patients 18 years of age and older.
Limitations of Use:
- Effectiveness of GIAZO in the treatment of female patients was not demonstrated in clinical trials [see Clinical Trials (14.1)].
- Safety and effectiveness of GIAZO therapy beyond 8 weeks have not been established.
- 2 DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
GIAZO is contraindicated in patients with hypersensitivity to salicylates, aminosalicylates or their metabolites, or to any of the components of GIAZO tablets [see Description (11)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Exacerbations of Ulcerative Colitis
Balsalazide is converted to mesalamine, which has been associated with an acute intolerance syndrome that may be difficult to distinguish from an exacerbation of ulcerative colitis. In controlled clinical trials with GIAZO in adults with ulcerative colitis, 7% of male patients reported exacerbation of the symptoms of ulcerative colitis. Symptoms include cramping, acute abdominal pain and bloody diarrhea, sometimes fever, headache, and rash. Observe patients closely for worsening of these symptoms while on treatment. If acute intolerance syndrome is suspected, promptly discontinue treatment with GIAZO.
5.2 Renal Impairment
Renal impairment, including minimal change nephropathy, acute and chronic interstitial nephritis and renal failure, has been reported in patients given products that release mesalamine in the gastrointestinal tract. Evaluate renal function prior to initiation of GIAZO therapy and periodically while on therapy. Exercise caution when using GIAZO in patients with known renal dysfunction or a history of renal disease.
5.3 Use in Hepatic Impairment
There have been reports of hepatic failure in patients with pre-existing liver disease who have been administered mesalamine. Because balsalazide is converted to mesalamine, use caution and consider liver function testing when administering GIAZO to patients with liver disease.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described below reflect exposure of GIAZO in 565 ulcerative colitis patients with mildly to moderately active disease. GIAZO was evaluated in one placebo-controlled trial (168 treated with GIAZO), one active-controlled trial (210 treated with GIAZO); and a subset of these patients also participated in an uncontrolled, open-label, extension study (additional 187 treated with GIAZO). The population studied had a mean age of 43.1 (range: 18-80) years; approximately 94% of patients were < 65 years old, 49% were male, and 84% were white.
In the placebo-controlled trial, the most common adverse reactions with GIAZO in male patients were headache, nasopharyngitis, anemia, diarrhea, fatigue, pharyngolaryngeal pain, and urinary tract infection. 10% of patients in the GIAZO group and 13% of patients in the placebo group discontinued treatment due to an adverse reaction. The majority of adverse reactions were mild to moderate in severity. The most common serious adverse reactions in both the placebo and GIAZO groups were gastrointestinal disorders, which were mainly associated with symptoms of ulcerative colitis.
Adverse reactions occurring in at least 2% of male patients and at a rate numerically higher than placebo in the placebo-controlled trial are listed in Table 1.
Table 1: Adverse Reactions Experienced by at Least 2% of GIAZO –Treated Male Patients and at a Rate Numerically Greater than Placebo in a Placebo-Controlled Trial
Adverse Reaction
GIAZO 6.6 g/day
N=82
PLACEBO
N=37
Anemia
3.7%
0%
Diarrhea
3.7%
0%
Pharyngolaryngeal Pain
3.7%
0%
Urinary Tract Infection
3.7%
0%
Arthralgia
2.4%
0%
Insomnia
2.4%
0%
Musculoskeletal Pain
2.4%
0%
Data collected from all three trials (placebo-controlled, active-controlled, and open-label) showed that female patients reported adverse reactions more frequently than did male patients (76% and 66%, respectively).
The following adverse reactions, presented by body system, were reported by less than 1% of GIAZO-treated ulcerative colitis patients in controlled trials.
Cardiovascular and Vascular: increased blood pressure, increased heart rate
Dermatological: erythema nodosum, rash
Respiratory, Thoracic and Mediastinal Disorders: dyspnea
Gastrointestinal Disorders: abdominal pain, constipation, defecation urgency, diarrhea, dry mouth, hard feces, flatulence, gastroesophageal reflux disease, vomiting
Hepatobiliary Disorders: increased aspartate aminotransferase
Infections and Infestations: gastroenteritis, upper respiratory infection
Musculoskeletal and Connective Tissue Disorders: arthralgia, back pain, myalgia
Nervous System Disorders: dizziness, lethargy
General Disorders and Administrative Site Disorders: face edema, fatigue, malaise, pain, pyrexia, swelling
6.2 Postmarketing Experience
Because these reactions are reported voluntarily from a population of unknown size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These adverse reactions have been chosen for inclusion due to a combination of seriousness, frequency of reporting, or potential causal connection to products which contain or are metabolized to mesalamine, including balsalazide.
Cardiovascular and Vascular: myocarditis, pericarditis, vasculitis
Respiratory: alveolitis, pleural effusion, pneumonia (with and without eosinophilia)
Gastrointestinal: pancreatitis
Renal: interstitial nephritis, renal failure.
Hepatobiliary Disorders: elevated liver enzymes (AST, ALT, GGT, LDH, alkaline phosphatase), elevated bilirubin, jaundice, cholestatic jaundice, cirrhosis, hepatocellular damage including liver necrosis and liver failure, Kawasaki-like syndrome including hepatic dysfunction. Some of these cases were fatal.
Dermatological: alopecia, pruritus
-
7 DRUG INTERACTIONS
Based on in vitro studies, balsalazide and its metabolites [5‑aminosalicylic acid (5-ASA), N-acetyl-5-aminosalicylic acid (N-Ac-5-ASA), 4‑aminobenzoyl-ß-alanine (4-ABA), and N-acetyl-4-aminobenzoyl-ß-alanine (N‑Ac‑4‑ABA)] are not expected to inhibit the metabolism of other drugs that are substrates of CYP1A2, CYP2C9, CYP2C19, CYP2D6, or CYP3A4/5.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Published data from meta-analyses, cohort studies and case series on the use of mesalamine, the active moiety of GIAZO, during pregnancy have not reliably informed an association with mesalamine and major birth defects, miscarriage, or adverse maternal or fetal outcomes (see Data). There are adverse effects on maternal and fetal outcomes associated with ulcerative colitis in pregnancy (see Clinical Considerations). In animal reproduction studies, there were no adverse developmental effects observed after oral administration of balsalazide disodium in pregnant rats and rabbits during organogenesis at doses up to 2.4 and 4.7 times, respectively, the maximum recommended human dose (MRHD) (see Data). The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease-associated maternal and embryo/fetal risk
Published data suggest that increased disease activity is associated with the risk of developing adverse pregnancy outcomes in women with ulcerative colitis. Adverse pregnancy outcomes include preterm delivery (before 37 weeks of gestation), low birth weight (less than 2500 g) infants, and small for gestational age at birth.
Data
Human Data
Published data from meta-analyses, cohort studies and case series on the use of mesalamine, the active moiety of GIAZO, during early pregnancy (first trimester) and throughout pregnancy have not reliably informed an association of mesalamine and major birth defects, miscarriage, or adverse maternal or fetal outcomes. There is no clear evidence that mesalamine exposure in early pregnancy is associated with an increase risk in major congenital malformations, including cardiac malformations. Published epidemiologic studies have important methodological limitations which hinder interpretation of the data, including inability to control for confounders, such as underlying maternal disease, and maternal use of concomitant medications, and missing information on the dose and duration of use for mesalamine products.
Animal Data
Reproduction studies in rats and rabbits following administration of balsalazide disodium during organogenesis at oral doses up to 2 g/kg/day, equivalent to 2.5 and 4.9 times the recommended human dose, respectively, based on body surface area, revealed no evidence of no adverse embryofetal developmental effects due to balsalazide disodium.
8.2 Lactation
Risk Summary
Data from published literature report the presence of mesalamine and its metabolite, N acetyl-5 aminosalicylic acid, in human milk in small amounts with relative infant doses (RID) of 0.1% or less for mesalamine (see Data). There are case reports of diarrhea in breastfed infants exposed to mesalamine (see Clinical Considerations). There is no information on the effects of the drug on milk production. The lack of clinical data during lactation precludes a clear determination of the risk of GIAZO to an infant during lactation; therefore, the developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for GIAZO and any potential adverse effects on the breastfed child from GIAZO or from the underlying maternal condition.
Clinical Considerations
Advise the caregiver to monitor breastfed infants for diarrhea.
Data
In published lactation studies, maternal mesalamine doses from various oral and rectal mesalamine formulations and products ranged from 500 mg to 4.8 g daily. The average concentration of mesalamine in milk ranged from non-detectable to 0.5 mg/L. The average concentration of N-acetyl-5-aminosalicylic acid in milk ranged from 0.2 to 9.3 mg/L. Based on these concentrations, estimated infant daily dosages for an exclusively breastfed infant are 0 to 0.075 mg/kg/day (RID 0 to 0.1%) of mesalamine and 0.03 to 1.4 mg/kg/day of N-acetyl-5-aminosalicylic acid.
8.4 Pediatric Use
Safety and effectiveness of GIAZO in pediatric patients have not been established.
8.5 Geriatric Use
Reports from uncontrolled clinical studies and postmarketing reporting systems suggested a higher incidence of blood dyscrasias, i.e., neutropenia and pancytopenia, in patients who were 65 years or older who were taking mesalamine-containing products. GIAZO is converted into mesalamine in the colon. Caution should be taken to closely monitor blood cell counts during therapy.
Clinical trials of GIAZO did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently than younger subjects. Other reported clinical experience has not identified differences in responses between elderly and younger patients. In general, the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy in elderly patients should be considered when prescribing GIAZO.
-
10 OVERDOSAGE
No case of overdose has been reported with GIAZO. GIAZO is an aminosalicylate, and symptoms of salicylate toxicity include: hematemesis, tachypnea, hyperpnea, tinnitus, deafness, lethargy, seizures, confusion, or dyspnea. Severe intoxication may lead to electrolyte and blood pH imbalance and potentially to other organ (e.g., renal and liver) involvement. There is no specific antidote for balsalazide overdose. Proper medical care should be sought immediately with appropriate supportive care, including the possible use of emesis, cathartics, and activated charcoal to prevent further absorption.
-
11 DESCRIPTION
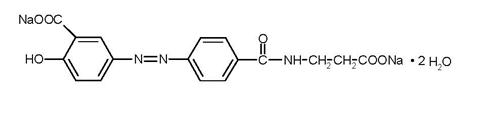
Each GIAZO tablet contains 1.1 g of balsalazide disodium, an orally available prodrug that is enzymatically cleaved to produce mesalamine (5‑aminosalicylic acid, 5‑ASA), an anti-inflammatory drug. Balsalazide disodium has the chemical name (E)-5-[[-4-[[(2-carboxyethyl) amino]carbonyl] phenyl]azo]-2-hydroxybenzoic acid, disodium salt, dihydrate. Its structural formula is:
Molecular Weight: 437.32
Molecular Formula: C17H13N3O6Na22H2O
Balsalazide disodium is a stable, odorless, orange to yellow, microcrystalline powder. It is insoluble in acid, but soluble at a pH of at least 4.5. It is freely soluble in water and isotonic saline, sparingly soluble in methanol and ethanol, and practically insoluble in all other organic solvents.
Inactive Ingredients: Each tablet contains hypromellose, magnesium stearate, and Opadry II Yellow. The sodium content of each tablet is approximately 126 mg.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Balsalazide is a prodrug of mesalamine (5-aminosalicylic acid, 5-ASA). The mechanism of action of 5-ASA is unknown, but appears to be local to the colonic mucosa rather than systemic. Mucosal production of arachidonic acid metabolites, both through the cyclooxygenase pathways, i.e., prostanoids, and through the lipoxygenase pathways, i.e., leukotrienes and hydroxyeicosatetraenoic acids, is increased in patients with ulcerative colitis, and it is possible that 5-ASA diminishes inflammation by blocking production of arachidonic acid metabolites in the colon.
12.3 Pharmacokinetics
Following oral administration, balsalazide is cleaved by azoreductases produced by anaerobic bacteria found in the gut, to release equimolar quantities of 5-ASA, the active moiety, and 4-aminobenzoyl-ß-alanine (4-ABA), a carrier moiety. Both of these moieties are N-acetylated to form N-Ac-5-ASA and N-Ac-4-ABA, respectively.
Absorption
After single-dose administration of 3.3 g GIAZO in 18 healthy subjects, the median time of peak plasma concentration (Tmax) was 0.5 hr for balsalazide, while the median Tmax was 12 hr for both 5-ASA and N-Ac-5-ASA (Table 2). Pharmacokinetic parameters exhibited high variability, with %CV ranging from 31% to 67% for AUC and from 27% to 68% for Cmax.
Pharmacokinetics were also estimated in healthy volunteers after repeated doses of 3.3 g GIAZO tablets every 12 hours for 7 days. After multiple doses, steady-state was achieved after about 3 days for balsalazide and all metabolites. The AUC and Cmax were the highest for N-Ac-5-ASA, followed by 5-ASA and balsalazide. There was minimal accumulation of balsalazide, as suggested by a 1.2-fold increase in AUC; however, a relatively larger increase in the systemic exposure to metabolites was observed at steady-state. The accumulation ratios based on AUC for the metabolites were 6.1 for 5-ASA, 3.6 for N-Ac-5-ASA, 4.8 for 4-ABA, and 3.6 for N-Ac-4-ABA.
Table 2: Pharmacokinetic Parameters for Balsalazide and Metabolites (5-ASA and N‑Ac-5-ASA) Following Single- and Repeated-Doses (every 12 hours) of 3.3 g Balsalazide Disodium as GIAZO (N=18)
Single Dose Repeated Dose Parameter Mean SD Mean SD Cmax (mcg/mL)
Balsalazide
0.3
0.2
0.3
0.2
5-ASA
0.5
0.3
1.5
0.6
N-Ac-5-ASA
1.2
0.4
2.2
0.6
Tmaxa (hr)
Balsalazide
0.5
(0.5-2)
0.5
(0.5-2)
5-ASA
12
(8-16)
12
(1.5-16)
N-Ac-5-ASA
12
(8-16)
10
(1-16)
AUCtau (mcghr/mL)
Balsalazide
1.3
0.7
1.6
0.9
5-ASA
2.2
1.6
13.4
6.3
N-Ac-5-ASA
5.9
2.9
21
6.4
AUC0‑∞ (mcghr/mL)
Balsalazide
1.4
0.8
NA
NA
5-ASA
8.5
3.9
NA
NA
N-Ac-5-ASA
33.5
14.1
NA
NA
T½b (hr)
Balsalazide
1.9
0.7
8.4
12.4
5-ASA
9.5b
10.1
9.0
8.6
N-Ac-5-ASA
10.4b
17.6
7.2
6.8
a Expressed as median and range.
b N=17
Effect of Food
After administration of single dose of 3.3 g (3 × 1.1 g tablets) of GIAZO with a high-fat meal in healthy volunteers, the AUC of balsalazide was unaffected compared to fasted administration, but the presence of food reduced both peak concentrations and AUC of the metabolites 5-ASA and N-Ac-5-ASA. A high fat meal increased the median Tmax for balsalazide from 0.5 to 2 hours; for 5-ASA from 12 to 24 hours; and for N-Ac-5-ASA from 12 to 24 hours. Under fed conditions, the mean Cmax was reduced by 44% for balsalazide, 65% for 5-ASA, and 48% for N-Ac-5-ASA. No significant changes were observed for AUC0-∞ for balsalazide; however, AUC0-∞ was reduced for 5-ASA by 46% and for N-Ac-5-ASA by 17%.
Distribution
The binding of balsalazide to human plasma proteins was ≥ 99%; 5-ASA and N-Ac-5-ASA were 43% and 78% bound, respectively, to plasma proteins.
Elimination
Metabolism
Following oral administration, balsalazide is cleaved by bacterial azoreduction to release equimolar quantities of 5-ASA, the active moiety, and 4-ABA, a carrier moiety. Mesalamine (5-ASA) and 4-ABA are further acetylated to N-Ac-5-ASA and N-Ac-4-ABA, respectively in the intestinal mucosa and liver. The terminal half-life was 1.9 hr for balsalazide, 9.5 h for 5-ASA, and 10.5 h for N-Ac-5-ASA.
Excretion
At steady-state following administration of repeated doses of 3.3 g GIAZO every 12 hours in healthy volunteers, the combined % of dose excreted in urine for balsalazide and its metabolites over 12 hours was 23%. The mean % of dose excreted in urine over 12 hours was 0.16% for balsalazide, 4.6% for 5-ASA, 15.6% for N-Ac-5-ASA, 0.40% for 4-ABA, and 1.8% for N-Ac-4-ABA.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 24-month rat (Sprague Dawley) carcinogenicity study, oral (dietary) balsalazide disodium at doses up to 2 g/kg/day was not tumorigenic. For a 50 kg person of average height this dose represents 2.5 times the recommended human dose on a body surface area basis. Balsalazide disodium was not genotoxic in the following in vitro or in vivo tests: Ames test, human lymphocyte chromosomal aberration test, and mouse lymphoma cell (L5178Y/TK+/-) forward mutation test, or mouse micronucleus test. However, it was genotoxic in the in vitro Chinese hamster lung cell (CH V79/HGPRT) forward mutation test.
The compound 4‑aminobenzoyl‑ß‑alanine, a metabolite of balsalazide disodium, was not genotoxic in the Ames test and the mouse lymphoma cell (L5178Y/TK+/-) forward mutation test but was positive in the human lymphocyte chromosomal aberration test. N-acetyl-4-aminobenzoyl-ß-alanine, a conjugated metabolite of balsalazide disodium, was not genotoxic in Ames test, the mouse lymphoma cell (L5178Y/TK+/-) forward mutation test, or the human lymphocyte chromosomal aberration test. Balsalazide disodium at oral doses up to 2 g/kg/day, 2.5 times the recommended human dose based on body surface area, was found to have no effect on fertility and reproductive performance in rats.
-
14 CLINICAL STUDIES
14.1 Ulcerative Colitis
A double-blind, placebo-controlled, multi-center trial was conducted in 250 adult patients with mildly to moderately active ulcerative colitis. The trial population was primarily white (84%), had a mean age of 44 years (7% age 65 years or older), and 49% were men. Disease activity was assessed using a modified Mayo Disease Activity Index1 (MMDAI), which was a sum of four subscores (bowel frequency, rectal bleeding, endoscopic appearance, and physician’s global assessment), each ranging from 0 to 3, with higher scores indicating worse disease. The median baseline MMDAI score was 8 and the median baseline rectal bleeding subscore was 2. Patients were randomized 2:1 to receive 8 weeks of treatment with either GIAZO 3.3 g twice daily or placebo.
The primary efficacy endpoint was the proportion of patients that achieved clinical improvement and improvement in the rectal bleeding subscale of the MMDAI at the end of 8 weeks of treatment. Clinical Improvement was defined as having both a ≥ 3 point improvement from baseline in the MMDAI score and a ≥ 1 point improvement from baseline in the rectal bleeding subscore. Two key secondary efficacy endpoints were the proportion of patients with Clinical Remission and Mucosal Healing at the end of 8 weeks of treatment. Clinical Remission was defined as a score of 0 for rectal bleeding and a combined score of ≤ 2 for bowel frequency and physician’s assessment using the MMDAI subscale; the endoscopic subscore was not considered in this definition. Mucosal Healing was defined as an endoscopy/sigmoidoscopy score of 0 or 1, where a score of 1 could include signs of erythema or decreased vascular pattern; by definition, the presence of friability indicated a score of 2 or 3.
After 8 weeks of treatment, the proportion of patients who met the definition of Clinical Improvement was greater for the GIAZO-treated group compared to the placebo group (Table 3).
Table 3: Proportion of Patients with Clinical Improvement* at Week 8
for the Total Population and by Gender Subgroups
GIAZO
Placebo
p-value
Total Population
55%
40%
0.0237
Males
Females
57%
54%
20%
58%
* Clinical Improvement: ≥ 3 improvement in MMDAI score and ≥ 1 point improvement in rectal bleeding.
These differences were statistically significant in the overall population; however, these effects were entirely driven by the results in the male subpopulation. With adjustment for multiplicity, statistically significant differences were also seen in the male patients for Clinical Remission (35% with GIAZO vs. 13% for placebo) and for Mucosal Healing (52% with GIAZO vs. 20% for placebo). Effectiveness of GIAZO was not demonstrated in the female subpopulation in the clinical trial.
- 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
GIAZO is available as oval, yellow, film-coated tablets containing 1.1 g balsalazide disodium, with “BZT” debossed on one side of the tablet.
Bottles of 180 tablets NDC: 65649-102-02
Storage
Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° and 30°C (59° and 86°F). [see USP Controlled Room Temperature].
-
17 PATIENT COUNSELING INFORMATION
- Instruct patients not to take GIAZO if they have a hypersensitivity to salicylates (e.g., aspirin).
- Instruct patients to take GIAZO with or without food.
- Advise patients who need to control sodium intake that the recommended dosing of GIAZO (6.6 g/day) provides about 756 mg of sodium per day.
- Instruct patients to contact their health care provider if they experience a worsening of their ulcerative colitis symptoms, because it could be due to a reaction to GIAZO.
-
Instruct patients to make sure they let their health care provider know:
- If they have or are later diagnosed with renal dysfunction. Damage to the kidney has been observed in some people given medications similar to GIAZO.
- If they have or are later diagnosed with liver disease. Worsening liver disease has been observed in some people given medications similar to GIAZO.
Manufactured for:
Salix Pharmaceuticals, a division of Bausch Health US, LLC
Bridgewater, NJ 08807 USA
U.S. Patent Numbers. 7,452,872; 7,625,884; 8,497,256; and 9,192,616
GIAZO is a trademark of Bausch Health Companies Inc. or its affiliates.
©2019 Bausch Health Companies Inc. or its affiliates.
9511700
70011736
- PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - Giazo 180 Tablets, Bottle Label
-
INGREDIENTS AND APPEARANCE
GIAZO
balsalazide disodium tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 65649-102 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BALSALAZIDE DISODIUM (UNII: 1XL6BJI034) (BALSALAZIDE - UNII:P80AL8J7ZP) BALSALAZIDE DISODIUM 1.1 g Inactive Ingredients Ingredient Name Strength HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color YELLOW Score no score Shape OVAL Size 19mm Flavor Imprint Code BZT Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65649-102-02 180 in 1 BOTTLE; Type 0: Not a Combination Product 02/03/2012 08/31/2020 2 NDC: 65649-102-01 6 in 1 BOTTLE; Type 0: Not a Combination Product 02/03/2012 02/28/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022205 02/03/2012 08/31/2020 Labeler - Salix Pharmaceuticals, Inc (793108036) Establishment Name Address ID/FEI Business Operations Nexgen Pharma, Inc. 160356114 MANUFACTURE(65649-102)
Trademark Results [GIAZO]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 GIAZO 97137848 not registered Live/Pending |
Pharma Trademarks Inc. 2021-11-22 |
 GIAZO 85356973 4305933 Dead/Cancelled |
Salix Pharmaceuticals, Inc. 2011-06-27 |
 GIAZO 77241937 not registered Dead/Abandoned |
Salix Pharmaceuticals, Inc. 2007-07-30 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.