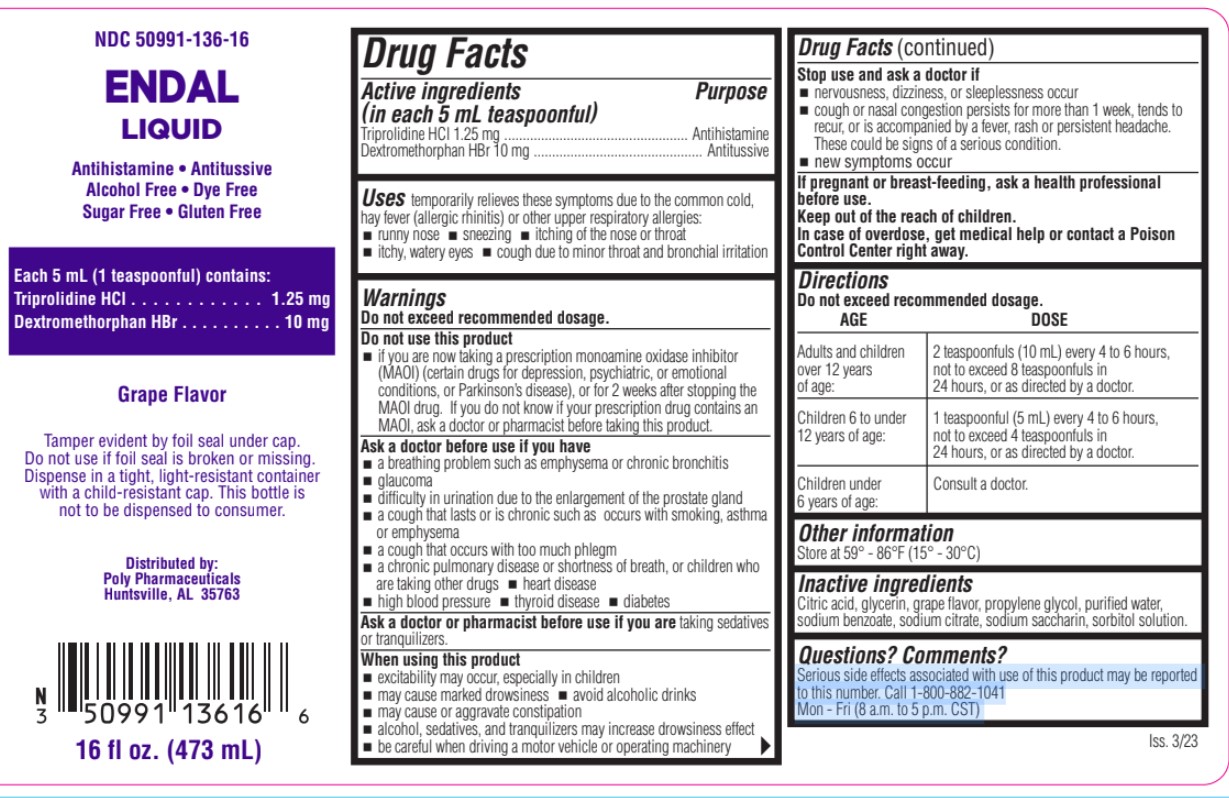
ENDAL- triprolidine hcl, dextromethorphan hbr liquid
Endal by
Drug Labeling and Warnings
Endal by is a Otc medication manufactured, distributed, or labeled by Poly Pharmaceuticals, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active Ingredient
- Purpose
- Uses
-
Warnings
Do not exceed recommended dosage.
Do not use this product
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- difficulty in urination due to the enlargement of the prostate gland
- a cough that lasts or is chronic such as occurs with smoking, asthma or emphysema
- a cough that occurs with too much phlegm
- a chronic pulmonary disease or shortness of breath, or children who are taking other drugs
- heart disease
- high blood pressure
- thyroid disease
- diabetes
When using this product
- excitability may occur, especially in children
- may cause marked drowsiness
- avoid alcoholic drinks
- may cause or aggravate constipation
- alcohol, sedatives, and tranquilizers may increase drowsiness effect
-
Directions
AGE DOSE Adults and children over 12 years of age 2 teaspoonfuls (10 mL) every 4 to 6 hours,
over 12 years not to exceed 8 teaspoonfuls in
of age: 24 hours, or as directed by a doctor.Children 6 to under 12 years of age 1 teaspoonful (5 mL) every 4 to 6 hours,
12 years of age: not to exceed 4 teaspoonfuls in
24 hours, or as directed by a doctor.Children under 6 years of age Consult a doctor. - Other Information
- Inactive Ingredients
- Questions? Comments?
- Package Label
-
INGREDIENTS AND APPEARANCE
ENDAL
triprolidine hcl, dextromethorphan hbr liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 50991-136 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRIPROLIDINE HYDROCHLORIDE (UNII: YAN7R5L890) (TRIPROLIDINE - UNII:2L8T9S52QM) TRIPROLIDINE HYDROCHLORIDE 1.25 mg in 5 mL DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 10 mg in 5 mL Inactive Ingredients Ingredient Name Strength SORBITOL SOLUTION (UNII: 8KW3E207O2) CITRIC ACID ACETATE (UNII: DSO12WL7AU) GLYCERIN (UNII: PDC6A3C0OX) SODIUM CITRATE (UNII: 1Q73Q2JULR) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SACCHARIN SODIUM (UNII: SB8ZUX40TY) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50991-136-16 473 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/22/2023 2 NDC: 50991-136-15 15 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/22/2023 3 NDC: 50991-136-05 5 mL in 1 BOTTLE; Type 0: Not a Combination Product 10/29/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 05/22/2023 Labeler - Poly Pharmaceuticals, Inc. (198449894)
Trademark Results [Endal]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ENDAL 97585638 not registered Live/Pending |
Poly Pharmaceuticals, Inc. 2022-09-09 |
 ENDAL 76160636 2509656 Live/Registered |
STAYMA CONSULTING SERVICES, LLC 2000-11-07 |
 ENDAL 73200274 not registered Dead/Abandoned |
UAD LABORATORIES, INC. 1979-01-16 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.