PureGel Hand Sanitizer, Citrus Fragrance - produced for Vaask, LLC (Vaask Moisturizing Hand Sanitizer)
Vaask Moisturizing Hand Sanitizer by
Drug Labeling and Warnings
Vaask Moisturizing Hand Sanitizer by is a Otc medication manufactured, distributed, or labeled by Vaask, LLC, Economy Polymers and Chemicals. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
VAASK MOISTURIZING HAND SANITIZER- ethyl alcohol gelÂ
Vaask, LLC
----------
PureGel Hand Sanitizer, Citrus Fragrance - produced for Vaask, LLC (Vaask Moisturizing Hand Sanitizer)
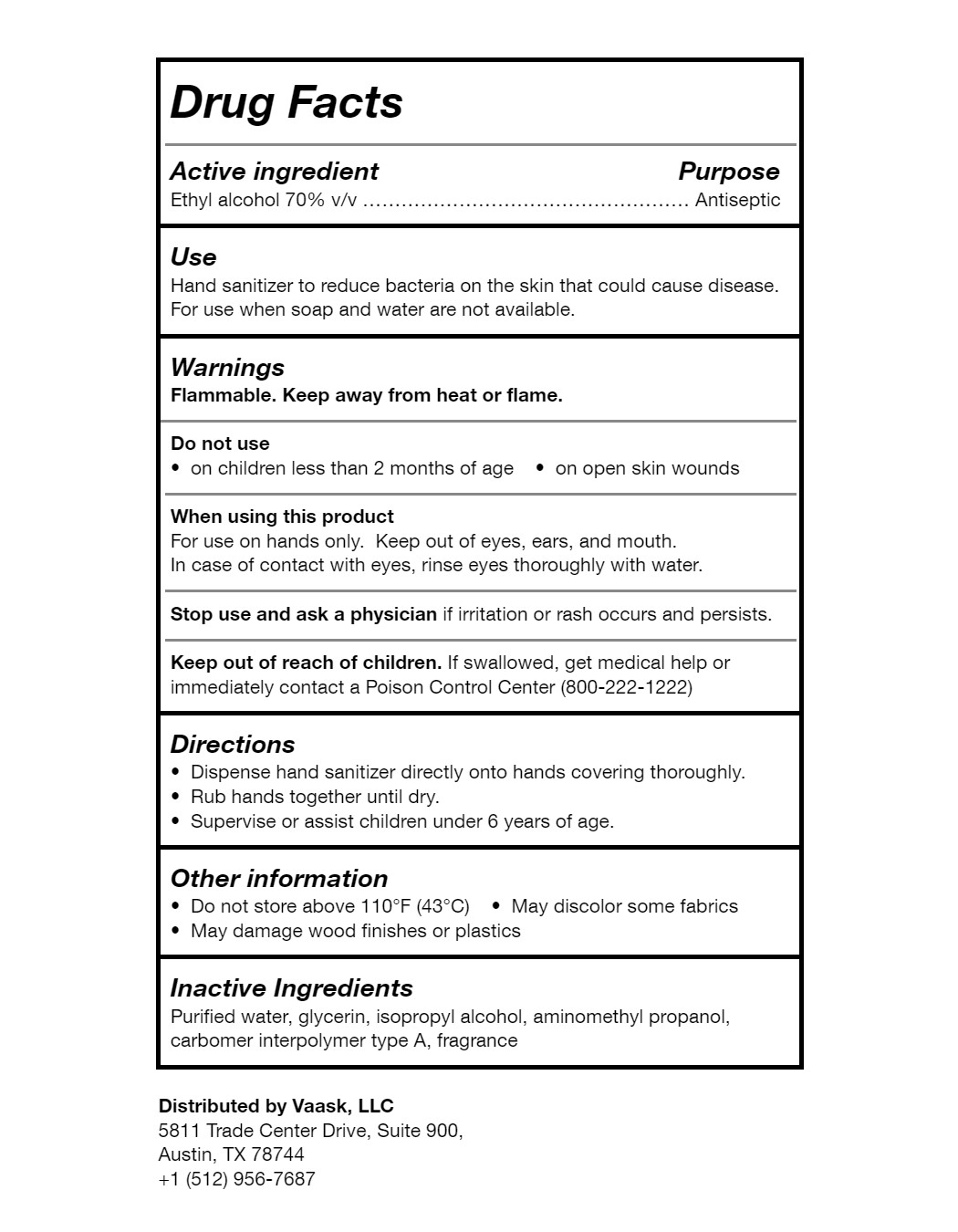
Use
Hand sanitizer to reduce bacteria on the skin that could cause disease. For use when soap and water are not available.
Warnings
FLAMMABLE. Keep away from heat or flame.
Directions
- Dispense hand sanitizer directly onto hands covering thoroughly
- Rub hands together until dry
- Supervise or assist children under 6 years of age
Other information
- Do not store over 110°F (43°C)
- May discolor some fabrics
- May damage wood finishes or plastics
| VAASK MOISTURIZING HAND SANITIZERÂ
ethyl alcohol gel |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler -Â Vaask, LLC (103829204) |
| Registrant -Â Economy Polymers and Chemicals (008087603) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Economy Polymers and Chemicals | 008087603 | manufacture(81625-548) | |
Revised: 12/2024
Â
Document Id: 29b85e51-7b73-18c0-e063-6294a90a4161
Set id: fc7114ad-750d-c4ce-e053-6294a90a69c4
Version: 2
Effective Time: 20241220
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.