KERENDIA- finerenone tablet, film coated KERENDIA- finerenone tablet
Kerendia by
Drug Labeling and Warnings
Kerendia by is a Prescription medication manufactured, distributed, or labeled by Bayer Healthcare Pharmaceuticals Inc., Bayer AG, Sharp Corporation, Bayer, AG. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use KERENDIA safely and effectively. See full prescribing information for KERENDIA.
KERENDIA (finerenone) tablets, for oral use
Initial U.S. Approval: 2021RECENT MAJOR CHANGES
INDICATIONS AND USAGE
Kerendia is a non-steroidal mineralocorticoid receptor antagonist (nsMRA) indicated to reduce the risk of:
- sustained estimated glomerular filtration rate (eGFR) decline, end stage kidney disease, cardiovascular death, non-fatal myocardial infarction, and hospitalization for heart failure in adult patients with chronic kidney disease (CKD) associated with type 2 diabetes (T2DM). (1)
- cardiovascular death, hospitalization for heart failure, and urgent heart failure visits in adult patients with heart failure with left ventricular ejection fraction (LVEF) ≥ 40% (1)
DOSAGE AND ADMINISTRATION
- The recommended starting dosage is 10 mg or 20 mg orally once daily based on eGFR and serum potassium thresholds. (2.1)
- Increase dosage after 4 weeks to the target dose of 20 mg once daily for CKD and T2DM based on eGFR and serum potassium thresholds. (2.3)
- Increase dosage after 4 weeks to the target dose of 20 mg or 40 mg once daily for HF with LVEF ≥ 40% based on eGFR and serum potassium thresholds. (2.3)
- Tablets may be taken with or without food (2.2)
DOSAGE FORMS AND STRENGTHS
Tablets: 10 mg, 20 mg, and 40 mg (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Hyperkalemia. Patients with decreased kidney function and higher baseline potassium levels are at increased risk. Monitor serum potassium levels and adjust dose as needed. (2.1, 2.2, 2.3, 5.1)
- Worsening of Renal Function in Patients with Heart Failure. Measure eGFR and adjust dose as needed. (2.1, 2.3, 6.1)
ADVERSE REACTIONS
Adverse reactions occurring in ≥ 1% of patients on Kerendia and more frequently than placebo are hyperkalemia, hypotension, and hyponatremia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Bayer HealthCare Pharmaceuticals Inc. at 1-888-842-2937 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Strong CYP3A4 Inhibitors: Use is contraindicated. (7.1)
- Grapefruit or grapefruit juice: Avoid concomitant use. (7.1)
- Moderate or weak CYP3A4 Inhibitors: Monitor serum potassium during drug initiation or dosage adjustment of either Kerendia or the moderate or weak CYP3A4 inhibitor, and adjust Kerendia dosage as appropriate (7.1)
- Strong or moderate CYP3A4 Inducers: Avoid concomitant use. (7.1)
- Sensitive CYP2C8 substrates at Kerendia 40 mg: Monitor more frequently for adverse reactions. (7.2)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 8/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Prior to Initiation of Kerendia
2.2 Recommended Starting Dosage
2.3 Monitoring and Dosage Adjustment
2.4 Missed Doses
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hyperkalemia
5.2 Worsening of Renal Function in Patients with Heart Failure
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on Kerendia
7.2 Effect of Kerendia on Other Drugs
7.3 Drugs That Affect Serum Potassium
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 CKD associated with T2DM
14.2 Heart Failure (LVEF ≥40%)
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
Kerendia is indicated to reduce the risk of:
- sustained estimated glomerular filtration rate (eGFR) decline, end-stage kidney disease, cardiovascular death, non-fatal myocardial infarction, and hospitalization for heart failure in adult patients with chronic kidney disease (CKD) associated with type 2 diabetes (T2DM).
- cardiovascular death, hospitalization for heart failure, and urgent heart failure visits in adult patients with heart failure with left ventricular ejection fraction (LVEF) ≥ 40%.
-
2 DOSAGE AND ADMINISTRATION
2.1 Prior to Initiation of Kerendia
Measure serum potassium levels and eGFR before initiation. Do not initiate treatment if serum potassium is > 5.0 mEq/L [see Warnings and Precautions (5.1)].
2.2 Recommended Starting Dosage
The recommended starting dose of Kerendia is based on eGFR and is presented in Table 1.
Table 1: Recommended Starting Dosage eGFR (mL/min/1.73m2) Starting Dose ≥ 60 20 mg orally once daily ≥ 25 to < 60 10 mg orally once daily < 25 Initiation is not recommended For patients who are unable to swallow whole tablets, Kerendia may be crushed and mixed with water or soft foods such as applesauce immediately prior to use and administered orally [see Clinical Pharmacology (12.3)].
2.3 Monitoring and Dosage Adjustment
CKD associated with T2DM
The target daily dose of Kerendia is 20 mg orally.
Measure serum potassium 4 weeks after initiating treatment and adjust dose (see Table 2); if serum potassium levels are > 4.8 to 5.0 mEq/L, initiation of Kerendia treatment may be considered with additional serum potassium monitoring within the first 4 weeks based on clinical judgment and serum potassium levels [see Warnings and Precautions (5.1)]. Measure serum potassium 4 weeks after a dose adjustment and periodically throughout treatment, and adjust the dose as needed (see Table 2) [see Warnings and Precautions (5.1) and Drug Interactions (7.1)].
Table 2: Dose Adjustment Based on Current Serum Potassium Concentration and Current Dose (CKD associated with T2DM) Current Kerendia Dose 10 mg once daily 20 mg once daily - * If eGFR has decreased by more than 30% compared to previous measurement, maintain 10 mg dose.
Current Serum Potassium (mEq/L) ≤ 4.8 Increase the dose to 20 mg once daily.* Maintain 20 mg once daily. > 4.8 – 5.5 Maintain 10 mg once daily. Maintain 20 mg once daily. > 5.5 Withhold Kerendia.
Consider restarting at 10 mg once daily when serum potassium ≤ 5.0 mEq/L.Withhold Kerendia.
Restart at 10 mg once daily when serum potassium ≤ 5.0 mEq/L.Heart Failure with LVEF ≥ 40%
The target daily dose of Kerendia for heart failure (LVEF ≥ 40%) is dependent on renal function (eGFR) at initiation of Kerendia treatment (see Table 3).
- The target daily dose is 40 mg orally once daily if eGFR at initiation is ≥ 60 mL/min/1.73m2.
- The target daily dose is 20 mg orally once daily if eGFR at initiation is ≥ 25 to < 60 mL/min/1.73m2.
Measure serum potassium and eGFR 4 weeks after initiating treatment and adjust dose (see Table 3). Measure serum potassium and eGFR 4 weeks after a dose adjustment and monitor periodically throughout treatment, and adjust the dose as needed (see Table 3) [see Warnings and Precautions (5.1 & 5.2) and Drug Interactions (7.1)].
Table 3: Dose Adjustment Based on Current Serum Potassium Concentration, eGFR, and Current Dose (Heart Failure (LVEF ≥ 40%)) Current Kerendia Dose 10 mg once daily 20 mg once daily 40 mg once daily - * If eGFR has decreased by more than 30% compared to previous measurement, maintain current dose.
- † If repeated serum potassium measurements are ≥5.5 mEq/L, restart Kerendia at 10 mg once daily when serum potassium < 5.0 mEq/L.
Current
Serum Potassium (mEq/L)< 5.0 Increase the dose to 20 mg once daily* Maintain 20 mg once daily if eGFR < 60 mL/min/1.73 m2 at initiation.
Otherwise increase the dose to 40 mg once daily*Maintain 40 mg once daily. ≥ 5.0 to < 5.5 Maintain current dose. ≥ 5.5 to < 6.0 Withhold Kerendia.
Restart at 10 mg once daily when serum potassium < 5.5 mEq/L.Decrease to 10 mg once daily. Decrease to 20 mg once daily. ≥ 6.0 Withhold Kerendia.
Restart at 10 mg once daily when serum potassium < 5.5 mEq/L.† - 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Kerendia is contraindicated in patients:
- Who are hypersensitive to any component of this product [see Adverse Reactions (6.2)].
- Who are receiving concomitant treatment with strong CYP3A4 inhibitors [see Drug Interactions (7.1)].
- With adrenal insufficiency.
-
5 WARNINGS AND PRECAUTIONS
5.1 Hyperkalemia
Kerendia can cause hyperkalemia [see Adverse Reactions (6.1)].
The risk for developing hyperkalemia increases with decreasing kidney function and is greater in patients with higher baseline potassium levels or other risk factors for hyperkalemia. Measure serum potassium and eGFR in all patients before initiation of treatment with Kerendia and dose accordingly [see Dosage and Administration (2.1)]. Do not initiate Kerendia if serum potassium is > 5.0 mEq/L.
Measure serum potassium periodically during treatment with Kerendia and adjust dose accordingly [see Dosage and Administration (2.3)]. More frequent monitoring may be necessary for patients at risk for hyperkalemia, including those on concomitant medications that impair potassium excretion or increase serum potassium [see Drug Interactions (7.1, 7.2)].
5.2 Worsening of Renal Function in Patients with Heart Failure
Kerendia can cause worsening of renal function in patients with heart failure. Rarely, severe events associated with worsening renal function, including events requiring hospitalization, have been observed [see Adverse Reactions (6.1)].
Measure eGFR in all patients before initiation of treatment or with dose titration of Kerendia and dose accordingly [see Dosage and Administration (2.1, 2.3)]. Initiation of Kerendia in patients with heart failure and an eGFR <25 mL/min/1.73m2 is not recommended.
Measure eGFR periodically during maintenance treatment with Kerendia in patients with heart failure. Consider delaying up-titration or interrupting treatment with Kerendia in patients who develop clinically significant worsening of renal function.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed elsewhere in the labeling:
- Hyperkalemia [see Warnings and Precautions (5.1)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
CKD associated with T2DM
The safety of Kerendia in patients with CKD associated with T2DM was evaluated in 2 randomized, double-blind, placebo-controlled, multicenter pivotal phase 3 studies, FIDELIO-DKD and FIGARO-DKD, in which a total of 6510 patients were treated with 10 or 20 mg once daily over a mean duration of 2.2 and 2.9 years, respectively.
Overall, serious adverse events occurred in 32% of patients receiving Kerendia and in 34% of patients receiving placebo in the FIDELIO-DKD study; the findings were similar in the FIGARO-DKD study. Permanent discontinuations due to adverse events also occurred in a similar proportion of patients in the two studies (6-7% of patients receiving Kerendia and in 5-6% of patients receiving placebo).
The most frequently reported (≥ 10%) adverse reaction was hyperkalemia [see Warnings and Precautions (5.1)]. Hospitalization due to hyperkalemia for the Kerendia group was 0.9% vs 0.2% in the placebo group across both studies. Hyperkalemia led to permanent discontinuation of treatment in 1.7% receiving Kerendia versus 0.6% of patients receiving placebo across both studies.
Table 4 shows adverse reactions that occurred more commonly on Kerendia than on placebo, and in at least 1% of patients treated with Kerendia.
Table 4: Adverse reactions reported in ≥ 1% of patients on Kerendia and more frequently than placebo (Pooled data from FIDELIO-DKD and FIGARO-DKD) Adverse reactions Kerendia
N = 6510
n (%)Placebo
N = 6489
n (%)Hyperkalemia 912 (14.0) 448 (6.9) Hypotension 302 (4.6) 194 (3.0) Hyponatremia 82 (1.3) 47 (0.7) Heart Failure with LVEF ≥ 40%
The safety of Kerendia in patients with heart failure (LVEF ≥40%) was evaluated in the randomized, double-blind, placebo-controlled, multicenter pivotal phase 3 study, FINEARTS-HF, in which a total of 2,993 patients were treated with 10 mg, 20 mg, or 40 mg once daily of Kerendia with a mean duration of treatment of 2.3 years.
The overall safety profile of Kerendia in the FINEARTS-HF study was largely consistent with the adverse reactions reported in patients with CKD and T2DM (Table 4). However, adverse reactions related to worsening renal function were reported more frequently in the Kerendia group (18%) compared with placebo (12%) in FINEARTS-HF. The most frequently reported adverse reactions included renal impairment (7% vs. 4%), eGFR decreased (5% vs. 4%), acute kidney injury (4% vs. 2%) and renal failure (3% vs. 2%). The majority of events were reported to be mild to moderate. These events led to dose modifications in 9% of patients receiving Kerendia versus 4% of patients receiving placebo. Hospitalization due to events related to worsening of renal function for the Kerendia group was 2.0% versus 1.3% in the placebo group.
Laboratory Test
Initiation of Kerendia may cause an initial small increase in blood creatinine levels (mean change <0.1 mg/dL) and a small decrease in eGFR (mean change 2-3 ml/min) that occurs within the first 4 weeks of starting therapy and then stabilizes. These changes were reversible after treatment discontinuation. Initiation of Kerendia may also cause a small increase in serum uric acid. This increase appears to attenuate over time.
6.2 Postmarketing Experience
The following additional adverse reactions have been reported in postmarketing experience with finerenone. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate their frequency reliably or to establish a causal relationship to drug exposure:
Hypersensitivity: Angioedema, Rash and Urticaria
-
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on Kerendia
Strong CYP3A4 Inhibitors
Kerendia is a CYP3A4 substrate. Concomitant use with a strong CYP3A4 inhibitor increases finerenone exposure [see Clinical Pharmacology (12.3)], which may increase the risk of Kerendia adverse reactions. Concomitant use of Kerendia with strong CYP3A4 inhibitors is contraindicated [see Contraindications (4)]. Avoid concomitant intake of grapefruit or grapefruit juice.
Moderate and Weak CYP3A4 Inhibitors
Kerendia is a CYP3A4 substrate. Concomitant use with a moderate or weak CYP3A4 inhibitor increases finerenone exposure [see Clinical Pharmacology (12.3)], which may increase the risk of Kerendia adverse reactions. Monitor serum potassium during drug initiation or dosage adjustment of either Kerendia or the moderate or weak CYP3A4 inhibitor, and adjust Kerendia dosage as appropriate [see Dosing and Administration (2.3) and Drug Interaction (7.2)].
Strong and Moderate CYP3A4 Inducers
Kerendia is a CYP3A4 substrate. Concomitant use of Kerendia with a strong or moderate CYP3A4 inducer decreases finerenone exposure [see Clinical Pharmacology (12.3)], which may reduce the efficacy of Kerendia. Avoid concomitant use of Kerendia with strong or moderate CYP3A4 inducers.
7.2 Effect of Kerendia on Other Drugs
CYP2C8 Substrates
Kerendia is a weak CYP2C8 inhibitor at 40 mg. Kerendia increases exposure of CYP2C8 substrates at 40 mg dose [see Clinical Pharmacology (12.3)], which may increase the risk of adverse reactions related to these substrates. Monitor patients more frequently for adverse reactions caused by sensitive CYP2C8 substrates if Kerendia 40 mg is co-administered with such substrates since minimal concentration changes may lead to serious adverse reactions.
7.3 Drugs That Affect Serum Potassium
More frequent serum potassium monitoring is warranted in patients receiving concomitant therapy with drugs or supplements that increase serum potassium. [see Dosage and Administration (2.3) and Warnings and Precautions (5.1)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on Kerendia use in pregnancy to evaluate for a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. Animal studies have shown developmental toxicity at exposures about 2 times those expected in humans (see Data). The clinical significance of these findings is unclear.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
In the embryo-fetal toxicity study in rats, finerenone resulted in reduced placental weights and signs of fetal toxicity, including reduced fetal weights and retarded ossification at the maternal toxic dose of 10 mg/kg/day corresponding to an AUCunbound of at least 7 times that in humans. At 30 mg/kg/day, the incidence of visceral and skeletal variations was increased (slight edema, shortened umbilical cord, slightly enlarged fontanelle) and one fetus showed complex malformations including a rare malformation (double aortic arch) at an AUCunbound of about 10 times that in humans at the 40 mg dose and about 25 times that in humans at the 20 mg dose. The doses free of any findings (low dose in rats, high dose in rabbits) provide safety margins of 4 to 5 times for the AUCunbound expected in humans.
When rats were exposed during pregnancy and lactation in the pre- and postnatal developmental toxicity study, increased pup mortality and other adverse effects (lower pup weight, delayed pinna unfolding) were observed at about 2 or 4 times the AUCunbound expected in humans at the dose of 40 mg and 20 mg, respectively. In addition, the offspring showed slightly increased locomotor activity, but no other neurobehavioral changes starting at about 2 or 4 times the AUCunbound expected in humans at the dose of 40 mg and 20 mg, respectively. The dose free of findings provides a safety margin of about 2 times for the AUCunbound expected in humans for the 20 mg dose and is in the therapeutic range for the 40 mg dose.
8.2 Lactation
Risk Summary
There are no data on the presence of finerenone or its metabolite in human milk, the effects on the breastfed infant or the effects of the drug on milk production. In a pre- and postnatal developmental toxicity study in rats, increased pup mortality and lower pup weight were observed at about 2 times the AUCunbound expected in humans. These findings suggest that finerenone is present in rat milk [see Use in Specific Populations (8.1) and Data]. When a drug is present in animal milk, it is likely that the drug will be present in human milk. Because of the potential risk to breastfed infants from exposure to Kerendia, avoid breastfeeding during treatment and for 1 day after treatment.
8.4 Pediatric Use
The safety and efficacy of Kerendia have not been established in patients below 18 years of age.
8.5 Geriatric Use
Of the 6510 patients who received Kerendia in the FIDELIO-DKD and FIGARO-DKD studies, 55% of patients were 65 years and older, and 14% were 75 years and older. Of the 2993 patients who received Kerendia in the FINEARTS study, 79% of patients were 65 years and older, and 43% were 75 years and older. No overall differences in safety or efficacy were observed between these patients and younger patients. No dose adjustment is required.
8.6 Hepatic Impairment
Avoid use of Kerendia in patients with severe hepatic impairment (Child Pugh C).
No dosage adjustment is recommended in patients with mild or moderate hepatic impairment (Child Pugh A or B).
Consider additional serum potassium monitoring in patients with moderate hepatic impairment (Child Pugh B) [see Dosing and Administration (2.3) and Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
In the event of suspected overdose, immediately interrupt Kerendia treatment. The most likely manifestation of overdose is hyperkalemia. If hyperkalemia develops, standard treatment should be initiated.
Finerenone is unlikely to be efficiently removed by hemodialysis given its fraction bound to plasma proteins of about 90%.
-
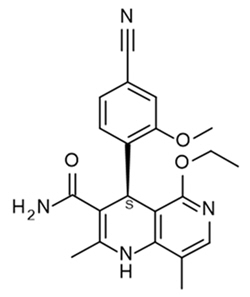
11 DESCRIPTION
Kerendia contains finerenone, a nonsteroidal mineralocorticoid receptor antagonist. Finerenone's chemical name is (4S)-4-(4-cyano-2- methoxyphenyl)-5-ethoxy-2,8-dimethyl-1,4-dihydro-1,6-naphthyridine-3-carboxamide. The molecular formula is C21H22N4O3 and the molecular weight is 378.43 g/mol. The structural formula is:
Finerenone is a white to yellow crystalline powder. It is practically insoluble in water; and sparingly soluble in 0.1 M HCl, ethanol, and acetone.
Each Kerendia tablet contains 10 mg, 20 mg, or 40 mg of finerenone. The inactive ingredients of Kerendia are lactose monohydrate, cellulose microcrystalline, croscarmellose sodium, hypromellose, magnesium stearate, and sodium lauryl sulfate. The film coating contains hypromellose, titanium dioxide and talc, in addition to ferric oxide red (10 mg and 40 mg strength tablets) or ferric oxide yellow (20 mg and 40 mg strength tablets).
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Finerenone is a nonsteroidal, selective antagonist of the mineralocorticoid receptor (MR), which is activated by aldosterone and cortisol and regulates gene transcription. Finerenone blocks MR mediated sodium reabsorption and MR overactivation in both epithelial (e.g., kidney) and nonepithelial (e.g., heart, and blood vessels) tissues. MR overactivation is thought to contribute to fibrosis and inflammation. Finerenone has a high potency and selectivity for the MR and has no relevant affinity for androgen, progesterone, estrogen, and glucocorticoid receptors.
12.2 Pharmacodynamics
In FIDELIO-DKD and FIGARO-DKD, randomized, double-blind, placebo-controlled, multicenter studies in adult patients with chronic kidney disease associated with type 2 diabetes, the placebo-corrected relative reduction in urinary albumin-to-creatinine ratio (UACR) in patients randomized to finerenone was 31% (95% CI 29-34%), and 32% (95% CI 30-35%) respectively at Month 4 and remained stable for the duration of the trial.
In ARTS DN, a randomized, double-blind, placebo-controlled, multicenter phase IIb dose finding study in adults with CKD and T2DM, the placebo-corrected relative reduction in UACR at Day 90 was 25% and 38% in patients treated with finerenone 10 mg and 20 mg once daily, respectively. In patients treated with Kerendia, the mean systolic blood pressure decreased by 3 mmHg and the mean diastolic blood pressure decreased by 1-2 mmHg at month 1, remaining stable thereafter.
12.3 Pharmacokinetics
Finerenone exposure increased proportionally over a dose range of 1.25 to 80 mg (0.06 to 4 times the maximum approved recommended dosage). Steady state of finerenone was achieved after 2 days of dosing. The estimated steady-state geometric mean Cmax,md was 166 µg/L and steady-state geometric mean AUCτ,md was 718 µg.h/L following administration of finerenone 20 mg to patients.
Absorption
Finerenone is completely absorbed after oral administration but undergoes metabolism resulting in absolute bioavailability of 44%. Finerenone Cmax was achieved between 0.5 and 1.25 hours after dosing.
Effect of Food
There was no clinically significant effect on finerenone AUC following administration with high fat, high calorie food.
Distribution
The volume of distribution at steady-state (Vss) of finerenone is 52.6 L. Plasma protein binding of finerenone is 92%, primarily to serum albumin, in vitro.
Elimination
The terminal half-life of finerenone is about 2 to 3 hours, and the systemic blood clearance is about 25 L/h.
Metabolism
Finerenone is primarily metabolized by CYP3A4 (90%) and to a lesser extent by CYP2C8 (10%) to inactive metabolites.
Excretion
About 80% of the administered dose is excreted in urine (<1% as unchanged) and approximately 20% in feces (< 0.2% as unchanged).
Specific Populations
There are no clinically significant effects of age (18 to 86 years), sex, race/ethnicity (White, Asian, Black, and Hispanic), or weight (54 to 126 kg) on the pharmacokinetics of finerenone.
Renal Impairment
There were no clinically relevant differences in finerenone AUC or Cmax values in patients with eGFR 15 to < 90 mL/min/1.73m2 compared to eGFR ≥ 90 mL/min/1.73 m2. For dosing recommendations based on eGFR and serum potassium levels see Dosage and Administration (2).
Hepatic Impairment
There was no clinically significant effect on finerenone exposure in cirrhotic patients with mild hepatic impairment (Child Pugh A).
Finerenone mean AUC was increased by 38% and Cmax was unchanged in cirrhotic patients with moderate hepatic impairment (Child Pugh B) compared to healthy control subjects.
The effect of severe hepatic impairment (Child Pugh C) on finerenone exposure was not studied.
Drug Interaction Studies
Clinical Studies and Model-Informed Approaches
Strong CYP3A Inhibitors: Concomitant use of itraconazole (strong CYP3A4 inhibitor) was predicted to increase finerenone AUC by >400%.
Moderate CYP3A Inhibitors: Concomitant use of erythromycin (moderate CYP3A4 inhibitor) increased finerenone mean AUC and Cmax by 248% and 88%, respectively. Concomitant use of verapamil (moderate CYP3A4 inhibitor) increased finerenone mean AUC and Cmax by 170% and 122%, respectively.
Weak CYP3A Inhibitors: Concomitant use of amiodarone (weak CYP3A4 inhibitor) increased finerenone AUC by 21%.
Strong or Moderate CYP3A Inducers: Concomitant use of efavirenz (moderate CYP3A4 inducer) and rifampicin (strong CYP3A4 inducer) was predicted to decrease finerenone AUC by 80% and 90%, respectively.
CYP3A4 Substrates: Concomitant use of multiple finerenone 40 mg doses once daily with midazolam (sensitive CYP3A4 substrate) increased the mean AUC by 31% with no effect on Cmax. There were no clinically significant differences in the pharmacokinetics of midazolam when used concomitantly with multiple finerenone 20 mg doses once daily.
CYP2C8 Substrates: Concomitant use of multiple finerenone 40 mg doses once daily with repaglinide (sensitive CYP2C8 substrate) increased the mean AUC and Cmax of repaglinide by 59% and 30%, respectively. There were no clinically significant differences in the pharmacokinetics of repaglinide when used concomitantly with multiple finerenone 20 mg doses once daily.
Other Drugs: No clinically significant differences in the pharmacokinetics of the following drugs were observed or predicted when used concomitantly with finerenone: S-warfarin (CYP2C9 substrate), digoxin (P-gp substrate), and rosuvastatin (BCRP and OATP substrate). There was no clinically significant difference in finerenone pharmacokinetics when used concomitantly with gemfibrozil (strong CYP2C8 inhibitor).
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Finerenone was non-genotoxic in an in vitro bacterial reverse mutation (Ames) assay, the in vitro chromosomal aberration assay in cultured Chinese hamster V79 cells, or the in vivo micronucleus assay in mice.
In 2-year carcinogenicity studies, finerenone did not show a statistically significant increase in tumor response in Wistar rats or in CD1 mice. In male mice, Leydig cell adenoma was numerically increased at a dose representing 10 times the AUCunbound in humans and is not considered clinically relevant. Finerenone did not impair fertility in male rats but impaired fertility in female rats at 9 times AUC to the maximum human exposure.
-
14 CLINICAL STUDIES
14.1 CKD associated with T2DM
FIDELIO-DKD (NCT: 02540993) and FIGARO-DKD (NCT: 02545049) studies were randomized, double-blind, placebo-controlled, multicenter studies in adult patients with chronic kidney disease (CKD) associated with type 2 diabetes (T2DM). In FIDELIO-DKD, patients needed to either have an UACR of 30 to < 300 mg/g, eGFR 25 to < 60 mL/min/1.73 m2 and diabetic retinopathy, or an UACR of ≥ 300 mg/g and an eGFR of 25 to < 75 mL/min/1.73 m2 to qualify for enrollment. In FIGARO-DKD, patients needed to have an UACR of 30 mg/g to < 300 mg/g and an eGFR of 25 to 90 mL/min/1.73m2, or an UACR ≥ 300 mg/g and an eGFR ≥ 60 mL/min/1.73m2.
Both trials excluded patients with known significant non-diabetic kidney disease. All patients were to have a serum potassium ≤ 4.8 mEq/L at screening and be receiving standard of care background therapy, including a maximum tolerated labeled dose of an angiotensin-converting enzyme inhibitor (ACEi) or angiotensin receptor blocker (ARB). Patients with a clinical diagnosis of chronic heart failure with reduced ejection fraction and persistent symptoms (New York Heart Association class II to IV) were excluded. The starting dose of Kerendia was based on screening eGFR (10 mg once daily in patients with an eGFR of 25 to < 60 mL/min/1.73 m2 and 20 mg once daily in patients with an eGFR ≥ 60 mL/min/1.73 m2). The dose of Kerendia could be titrated during the study, with a target dose of 20 mg daily.
The primary objective of the FIDELIO-DKD study was to determine whether Kerendia reduced the incidence of a sustained decline in eGFR of ≥ 40%, kidney failure (defined as chronic dialysis, kidney transplantation, or a sustained decrease in eGFR to < 15 mL/min/1.73 m2), or renal death. The secondary outcome was a composite of time to first occurrence of CV death, non-fatal MI, non-fatal stroke or hospitalization for heart failure. The primary objective of the FIGARO-DKD study was to determine whether Kerendia reduced the time to first occurrence of CV death, non-fatal MI, non-fatal stroke or hospitalization for heart failure. The secondary outcome was a composite of time to kidney failure, a sustained decline in eGFR of 40% or more compared to baseline over at least 4 weeks, or renal death.
In FIDELIO-DKD, a total of 5674 patients were randomized to receive Kerendia (N=2833) or placebo (N=2841) and were followed for a median of 2.6 years. The mean age of the study population was 66 years, and 70% of patients were male. This global trial population was 63% White, 25% Asian, and 5% Black. At baseline, the mean eGFR was 44 mL/min/1.73m2, with 55% of patients having an eGFR < 45 mL/min/1.73m2. Median urine albumin-to-creatinine ratio (UACR) was 852 mg/g, mean glycated hemoglobin A1c (HbA1c) was 7.7%, and the mean blood pressure was 138/76 mmHg. Approximately 46% of patients had a history of atherosclerotic cardiovascular disease and 8% had a history of heart failure. At baseline, 99.8% of patients were treated with an ACEi or ARB. Approximately 97% were on an antidiabetic agent (insulin [64.1%], biguanides [44%], glucagon-like peptide-1 [GLP-1] receptor agonists [7%], sodium-glucose cotransporter 2 [SGLT2] inhibitors [5%]), 74% were on a statin, and 57% were on an antiplatelet agent.
In FIGARO-DKD, a total of 7352 patients were randomized to receive Kerendia (N=3686) or placebo (N=3666) and were followed for 3.4 years. As compared to FIDELIO-DKD, baseline eGFR was higher in FIGARO-DKD (mean eGFR 68, with 62% of patients having an eGFR ≥ 60 mL/min/1.73 m2) and median UACR was lower (308 mg/g). Otherwise, baseline patient characteristics and background therapies were similar in the two trials.
In FIDELIO-DKD, Kerendia reduced the incidence of the primary composite endpoint of a sustained decline in eGFR of ≥ 40%, kidney failure, or renal death (HR 0.82, 95% CI 0.73-0.93, p=0.001) as shown in Table 5 and Figure 1. The treatment effect reflected a reduction in a sustained decline in eGFR of ≥ 40% and progression to kidney failure. There were few renal deaths during the trial. Kerendia also reduced the incidence of the secondary composite endpoint of cardiovascular (CV) death, non-fatal myocardial infarction (MI), non-fatal stroke or hospitalization for heart failure (HR 0.86, 95% CI 0.75-0.99, p=0.034) as shown in Table 5 and Figure 3. The treatment effect reflected a reduction in CV death, non-fatal MI, and hospitalization for heart failure. The treatment effect on the primary and secondary composite endpoints was generally consistent across subgroups.
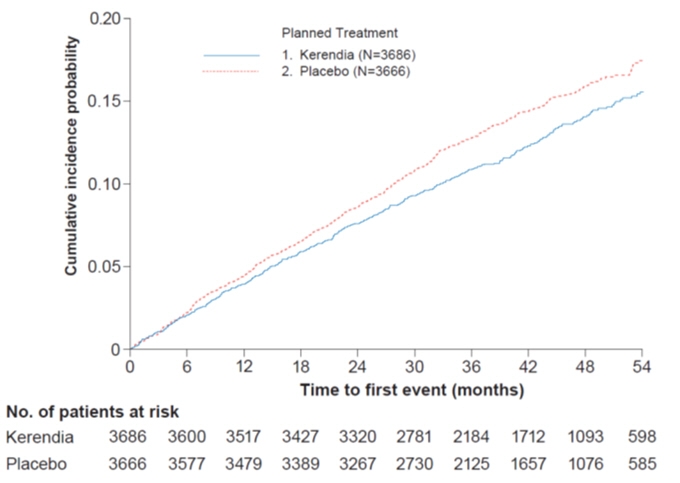
In FIGARO-DKD, Kerendia reduced the incidence of the primary composite endpoint of CV death, non-fatal MI, non-fatal stroke or hospitalization for heart failure (HR 0.87, 95% CI 0.76-0.98, p = 0.026) as shown in Table 5 and Figure 4. The treatment effect was mainly driven by an effect on hospitalization for heart failure, though CV death also contributed to the treatment effect. The treatment effect on the primary composite endpoint was generally consistent across subgroups, including patients with and without pre-existing cardiovascular disease. The findings for the renal composite endpoint are shown in Table 5 and Figure 2.
Table 5: Analysis of the Primary and Secondary Time-to-Event Endpoints (and their Individual Components) in Phase 3 Studies FIDELIO-DKD and FIGARO-DKD FIDELIO-DKD FIGARO-DKD Kerendia
N=2833Placebo
N=2841Treatment Effect
Kerendia / PlaceboKerendia
N=3686Placebo
N=3666Treatment Effect
Kerendia / PlaceboTime-to-event Endpoints: Event Rate (100 pt-yr) Event Rate (100 pt-yr) Hazard Ratio
(95% CI)p-value Event Rate (100 pt-yr) Event Rate (100 pt-yr) Hazard Ratio
(95% CI)p-value p-value: two-sided p-value from stratified logrank test
CI = confidence interval, CV = cardiovascular, eGFR = estimated glomerular filtration rate, MI = myocardial infarction, N = number of subjects, n = number of subjects with event, pt-yr = patient year.
NOTE: Time to first event was analyzed in a Cox proportional hazards model. For patients with multiple events, only the first event contributed to the composite endpoint. Sums of the numbers of first events for the single components do not add up to the numbers of events in the composite endpoint.Composite of kidney failure, sustained eGFR decline ≥40% or renal death 7.6 9.1 0.82
[0.73; 0.93]0.001 3.2 3.6 0.87
[0.76; 1.01]- Kidney failure 3.0 3.4 0.87
[0.72; 1.05]- 0.4 0.5 0.72
[0.49;1.05]- Sustained eGFR decline ≥40% 7.2 8.7 0.81
[0.72; 0.92]- 3.1 3.5 0.87
[0.75;>1.00]- Renal death - - - - - - - - Composite of CV death, non-fatal MI, non-fatal stroke or hospitalization for heart failure 5.1 5.9 0.86
[0.75; 0.99]0.034 3.9 4.5 0.87
[0.76; 0.98]0.026 CV death 1.7 2.0 0.86
[0.68;1.08]- 1.6 1.8 0.90
[0.73; 1.08]- Non-fatal MI 0.9 1.2 0.80
[0.58;1.09]- 0.9 0.8 0.99
[0.76; 1.32]- Non-fatal stroke 1.2 1.2 1.03
[0.76;1.38]- 0.9 0.9 0.97
[0.74; 1.26]- Hospitalization for heart failure 1.9 2.2 0.86
[0.68;1.08]- 1.0 1.4 0.71
[0.56; 0.90]- Figure 1: Time to first occurrence of kidney failure, sustained decline in eGFR ≥ 40% from baseline, or renal death in the FIDELIO-DKD study
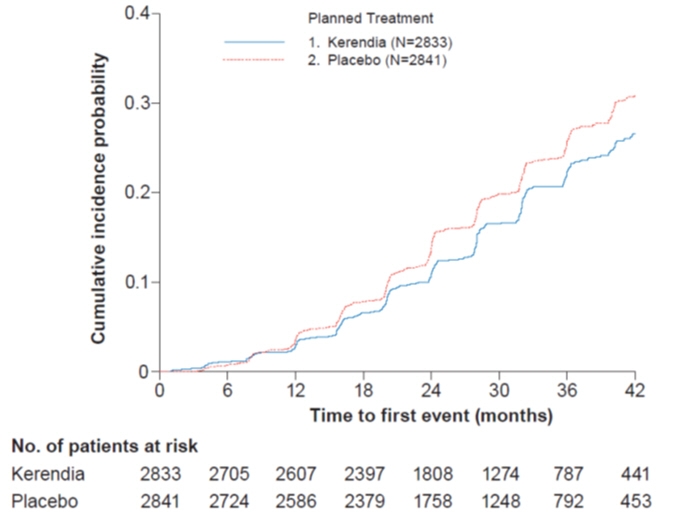
Figure 2: Time to first occurrence of kidney failure, sustained decline in eGFR ≥ 40% from baseline, or renal death in the FIGARO-DKD study
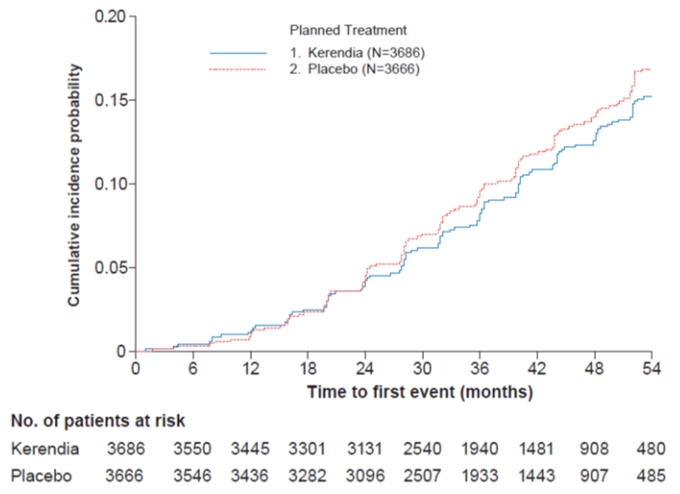
Figure 3: Time to first occurrence of CV death, non-fatal myocardial infarction, non-fatal stroke or hospitalization for heart failure in the FIDELIO-DKD study
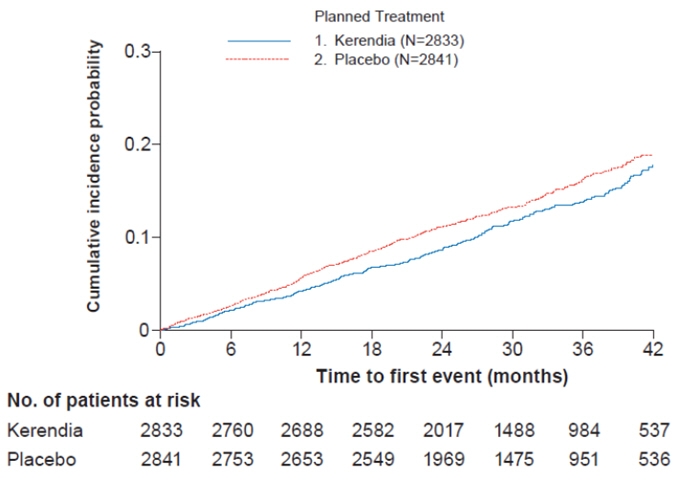
Figure 4: Time to first occurrence of CV death, non-fatal myocardial infarction, non-fatal stroke or hospitalization for heart failure in the FIGARO-DKD study
14.2 Heart Failure (LVEF ≥40%)
FINEARTS-HF (NCT: 04435626) was a randomized, double-blind, placebo-controlled, multicenter study in adult patients with heart failure (New York Heart Association [NYHA] class II–IV) with documented left ventricular ejection fraction (LVEF) ≥40%. Patients were required to have an eGFR ≥25 mL/min/1.73m2 and serum potassium ≤5.0 mEq/L at screening and randomization and were receiving background heart failure medical treatment, including diuretics. The primary endpoint was the composite of cardiovascular (CV) death and total (first and recurrent) heart failure events comprised of hospitalization for heart failure and urgent heart failure visits.
In FINEARTS-HF, 6001 patients were analyzed; 3003 were randomized to Kerendia and 2998 were randomized to placebo and were followed for a median of 2.7 years. The study included 3247 (54%) patients with a heart failure event in the past 3 months, including 1219 (20%) patients randomized during the hospitalization or within 7 days from the heart failure event. Patients with eGFR ≥ 25 to <60 mL/min/1.73m2 initiated Kerendia 10 mg and patients with eGFR ≥60 mL/min/1.73m2 initiated Kerendia 20 mg, and both groups were titrated up to a target dose of Kerendia 20 mg and 40 mg, respectively. At month 6, approximately 65% of the patients reached their target dose. The trial population was 79% White, 17% Asian, and 1.5% Black. The mean age at enrollment was 72 years and 46% of patients were female. At baseline, the mean LVEF was 53%, with 64% of patients having an LVEF ≥50%, and 69% and 30% of patients were NYHA class II and III, respectively. Mean blood pressure was 129/75 mmHg and mean body mass index (BMI) was 30 kg/m2. The median NT-proBNP was 1041 pg/mL, the mean eGFR was 62 mL/min/1.73m2 with 48% of patients having an eGFR <60 mL/min/1.73m2. Atrial fibrillation was present on screening/baseline ECG for 38% of patients and 41% had diabetes mellitus. The majority of patients were on loop diuretics (87%), an angiotensin-converting enzyme inhibitor (ACEi) or angiotensin receptor blocker (ARB) (79%), or an angiotensin receptor neprilysin inhibitor (ARNI) (9%), and 14% were on sodium-glucose cotransporter 2 (SGLT2) inhibitors.
Kerendia reduced the risk of the primary composite endpoint compared to placebo (Relative Risk [RR] 0.84, 95% CI 0.74-0.95, p=0.007). See Table 6 and Figure 5 below. The Kerendia and placebo event curves separated early and continued to diverge over the study period, see Figure 5 below. Kerendia reduced the risk of the secondary endpoint of total heart failure events (hospitalization for HF or urgent HF visit) compared to placebo (RR 0.82, 95% CI 0.71-0.94, p=0.006). The treatment effect for the primary endpoint was consistent across all pre-specified subgroups, including sex, LVEF, NYHA class, eGFR, time since latest heart failure event, SGLT2 inhibitor therapy, and diabetes mellitus status.
Table 6: Analysis of the Primary Endpoint (and its Individual Components) and Secondary Endpoint in Phase 3 Study FINEARTS-HF FINEARTS-HF Kerendia
10 or 20 or 40 mg OD
N=3003Placebo
N=2998Treatment Effect
Kerendia vs PlaceboPrimary Efficacy Outcome and Components: Value Event Rate
(100 pt–yr)Value Event Rate (100 pt–yr) Ratio or difference (95% CI) p-value E = total number of events; n = number of patients with an event ; RR = rate ratio; HR = hazard ratio; HF = Heart Failure; CV = Cardiovascular. - * Total HF events (hospitalization for HF or urgent HF visit) was a prespecified secondary endpoint.
Primary composite of CV death and total HF events – E (n; %) 1083
(624; 20.8%)14.9 1283
(719; 24.0%)17.7 RR 0.84
(0.74, 0.95)0.007 Total HF events – E (n; %)* 842
(479; 16.0%)11.6 1024
(573; 19.1%)14.1 RR 0.82
(0.71, 0.94)0.006 CV death – n (%) 242
(8.1%)3.3 260
(8.7%)3.6 HR 0.93
(0.78, 1.11)- Figure 5: Mean cumulative functions for primary composite endpoint of CV death and total HF events in Phase 3 study FINEARTS-HF
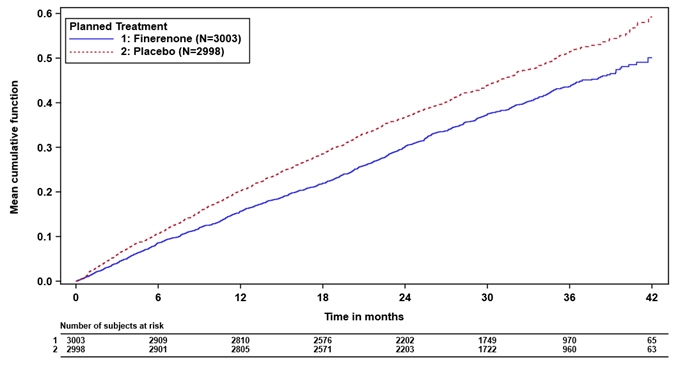
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Kerendia is available as a film-coated tablet in three strengths. The 10 mg is a pink oblong tablet with "FI" on one side of tablet and "10" on the other side of tablet. The 20 mg tablet is a yellow oblong tablet with "FI" on one side of tablet and "20" on the other side of tablet. The 40 mg tablet is a gray-orange oblong tablet with "FI" on one side of tablet and "40" on the other side of tablet.
Kerendia Tablets 10 mg Bottle of 30 tablets NDC: 50419-540-01 Bottle of 90 tablets NDC: 50419-540-02 Hospital Blister Pack of 30 tablets NDC: 50419-540-06 Kerendia Tablets 20 mg Bottle of 30 tablets NDC: 50419-541-01 Bottle of 90 tablets NDC: 50419-541-02 Hospital Blister Pack of 30 tablets NDC: 50419-541-06 Kerendia Tablets 40 mg Bottle of 30 tablets NDC: 50419-542-01 Bottle of 90 tablets NDC: 50419-542-02 Hospital Blister Pack of 30 tablets NDC: 50419-542-06 -
17 PATIENT COUNSELING INFORMATION
Advise patients of the need for periodic monitoring of serum potassium levels. Advise patients receiving Kerendia to consult with their physician before using potassium supplements or salt substitutes containing potassium [see Warnings and Precautions (5.1)].
Advise patients to avoid strong or moderate CYP3A4 inducers and to find alternative medicinal products with no or weak potential to induce CYP3A4 [see Drug Interactions (7.1)]
Avoid concomitant intake of grapefruit or grapefruit juice as it is expected to increase the plasma concentration of finerenone [see Drug Interactions (7.1)].
Advise women that breastfeeding is not recommended at the time of treatment with KERENDIA and for 1 day after treatment [see Use in Specific Populations (8.2)].
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL- 10 mg Tablet Carton
- PRINCIPAL DISPLAY PANEL- 20 mg Tablet Carton
- PRINCIPAL DISPLAY PANEL- 40 mg Tablet Carton
-
INGREDIENTS AND APPEARANCE
KERENDIA
finerenone tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 50419-540 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FINERENONE (UNII: DE2O63YV8R) (FINERENONE - UNII:DE2O63YV8R) FINERENONE 10 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) SODIUM LAURYL SULFATE (UNII: 368GB5141J) Product Characteristics Color PINK Score no score Shape OVAL Size 4mm Flavor Imprint Code 10;FI Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50419-540-01 30 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/09/2021 2 NDC: 50419-540-02 90 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/09/2021 3 NDC: 50419-540-70 7 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/09/2021 4 NDC: 50419-540-06 10 in 1 BLISTER PACK; Type 0: Not a Combination Product 08/23/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA215341 07/09/2021 KERENDIA
finerenone tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 50419-541 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FINERENONE (UNII: DE2O63YV8R) (FINERENONE - UNII:DE2O63YV8R) FINERENONE 20 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) SODIUM LAURYL SULFATE (UNII: 368GB5141J) Product Characteristics Color YELLOW Score no score Shape OVAL Size 4mm Flavor Imprint Code 20;FI Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50419-541-01 30 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/09/2021 2 NDC: 50419-541-02 90 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/09/2021 3 NDC: 50419-541-70 7 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/09/2021 4 NDC: 50419-541-06 10 in 1 BLISTER PACK; Type 0: Not a Combination Product 08/23/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA215341 07/09/2021 KERENDIA
finerenone tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 50419-542 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FINERENONE (UNII: DE2O63YV8R) (FINERENONE - UNII:DE2O63YV8R) FINERENONE 40 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) SODIUM LAURYL SULFATE (UNII: 368GB5141J) Product Characteristics Color ORANGE (gray-orange) Score no score Shape OVAL Size 11mm Flavor Imprint Code 40;FI Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50419-542-01 30 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/11/2025 2 NDC: 50419-542-02 90 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/11/2025 3 NDC: 50419-542-70 7 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/11/2025 4 NDC: 50419-542-06 10 in 1 BLISTER PACK; Type 0: Not a Combination Product 07/11/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA215341 07/09/2021 Labeler - Bayer HealthCare Pharmaceuticals Inc. (005436809) Registrant - Bayer AG (314947622) Establishment Name Address ID/FEI Business Operations Bayer AG 314947622 MANUFACTURE(50419-540, 50419-541, 50419-542) , ANALYSIS(50419-540, 50419-541, 50419-542) , PARTICLE SIZE REDUCTION(50419-540, 50419-541, 50419-542) , PACK(50419-540, 50419-541, 50419-542) Establishment Name Address ID/FEI Business Operations Sharp Corporation 143696495 PACK(50419-540, 50419-541, 50419-542) , LABEL(50419-540, 50419-541, 50419-542) Establishment Name Address ID/FEI Business Operations Bayer AG 323208116 API MANUFACTURE(50419-540, 50419-541, 50419-542) , ANALYSIS(50419-540, 50419-541, 50419-542) Establishment Name Address ID/FEI Business Operations Bayer AG 342872971 PARTICLE SIZE REDUCTION(50419-540, 50419-541, 50419-542) , PACK(50419-540, 50419-541, 50419-542) Establishment Name Address ID/FEI Business Operations Bayer AG 314398484 ANALYSIS(50419-540, 50419-541, 50419-542) Establishment Name Address ID/FEI Business Operations AMPAC Fine Chemicals, Rancho Cordova 073903937 API MANUFACTURE(50419-540, 50419-541, 50419-542) Establishment Name Address ID/FEI Business Operations Delpharm Milano Srl 437999026 LABEL(50419-540, 50419-541, 50419-542) , PACK(50419-540, 50419-541, 50419-542)
Trademark Results [Kerendia]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 KERENDIA 88432876 5931047 Live/Registered |
Bayer Aktiengesellschaft 2019-05-16 |
 KERENDIA 87700817 5518140 Live/Registered |
Bayer Aktiengesellschaft 2017-11-29 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.