AYRADIA- metronidazole solution
AYRADIA by
Drug Labeling and Warnings
AYRADIA by is a Animal medication manufactured, distributed, or labeled by Virbac AH Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- GENERAL PRECAUTIONS
-
Description
AYRADIA™ (metronidazole oral suspension) for dogs contains metronidazole USP. Metronidazole is a nitroimidazole, in the drug class anti-infectives, with anti-bacterial and anti-protozoal activities. The product is a flavored oily suspension with brown visible particles. The chemical composition of metronidazole is 2-(2-methyl-5-nitroimidazol-1-yl) ethanol. The empirical formula of metronidazole is: C6H9N3O3.
AYRADIA oral suspension contains 125 mg metronidazole/mL in a flavored, medium-chain triglyceride, liquid base.
- Indication
-
Dosage and Administration
Shake vigorously before use.
AYRADIA oral suspension is administered orally at a dose of 25 mg/kg (11.3 mg/lb) of body weight, using the supplied syringe, twice daily for five consecutive days. Each line on the included dosing syringe represents 0.1 mL of oral suspension. For dogs weighing more than 15 kg (33 lb), the total dose volume will be divided over multiple syringe draws because the dosing syringe only holds up to 3 mL. Alternatively, a standard luer lock syringe that holds more than 3 mL can also be used. The flavored suspension can be administered directly into the mouth or in a small amount of food (see Instructions for Using the Dispensing System and Preparing a Dose of AYRADIA oral suspension on reverse side). - Contraindications
-
Warnings
User Safety Warnings
Keep out of reach of children. Not for use in humans. Metronidazole has been found to cause cancer in laboratory animals; however, there is inadequate evidence of carcinogenicity in humans. People with known sensitivity to metronidazole or other nitroimidazole derivatives should avoid contact with AYRADIA oral suspension. This product is not a dermal or eye irritant but is a skin sensitizer which can potentially cause allergic contact dermatitis. Wash hands after use. Avoid contact with skin. In case of skin contact, wash the affected area thoroughly. Persons who come in contact with the dog’s saliva during the first five minutes after administration should wash their hands. If the drug has been applied to dog food, the dog food should be kept away from children until after the dog has finished eating. Avoid accidental ingestion. In case of accidental ingestion, seek medical advice immediately.
Animal Safety Warnings
Federal law prohibits the extra-label use of this drug in food-producing animals.Keep AYRADIA oral suspension in a secure location out of reach of dogs, cats, and other animals to prevent accidental ingestion or overdose.
-
Precautions
Prescribing antimicrobial drugs in the absence of a proven or strongly suspected infection is unlikely to provide benefit to treated animals and may increase the risk of the development of drug resistant animal pathogens.
Use with caution in dogs with hepatic dysfunction.
Adverse neurologic effects have been associated with AYRADIA oral suspension use at high doses (see Target Animal Safety), but individual variation in sensitivity of dogs to the adverse neurologic effects of metronidazole has been reported.
The safe use of this drug in dogs intended for breeding purposes and in pregnant or lactating bitches has not been evaluated.
-
Adverse Reactions
In a clinical field effectiveness and safety study, 120 dogs were treated with AYRADIA oral suspension and 60 dogs were treated with a vehicle control. The most frequently reported adverse reactions were diarrhea (6.7% treated, 5% vehicle) and vomiting (4.2% treated, 3.3% vehicle). One dog treated with AYRADIA oral suspension was reported to have hyperactivity while being treated.
The safety of AYRADIA oral suspension was also evaluated in a masked, active-controlled, multi-site field study, to evaluate the effectiveness of AYRADIA oral suspension for the treatment of Giardia spp. in dogs. Enrollment included 180 client-owned dogs diagnosed with Giardia spp. infection; 91 dogs were treated with AYRADIA oral suspension at 25 mg/kg twice daily for 5 consecutive days and 89 were treated with an active control. The dogs were 7.8 weeks to 13.4 years old, various pure or mixed breeds, and intact or neutered male dogs or intact or spayed female dogs. The most frequently reported adverse reactions in dogs treated with AYRADIA oral suspension were vomiting (14.3%) and diarrhea (3.3%). Other less frequently reported (<1.2%) adverse reactions included hypersalivation, abdominal pain, polydipsia and polyuria, anorexia, otitis externa, and lethargy.
Contact Information
To report suspected adverse drug events, for technical assistance or to obtain a copy of the Safety Data Sheet (SDS), contact Virbac AH, Inc. at 1-800-338-3659 or us.virbac.com. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or http://www.fda.gov/reportanimalae -
Clinical Pharmacology
Mechanism of Action
Metronidazole is a nitroimidazole compound known to exert antiprotozoal and antibacterial activity. Metronidazole has antiprotozoal activity against Giardia duodenalis. The mechanism of action for its antiprotozoal activity is not well understood but it acts primarily against the trophozoite forms of the parasites resulting in a decrease in cyst shedding. Metronidazole is reduced as it enters the susceptible target cell where it interacts with bacterial or protozoal DNA, causing a loss of helical structure and strand breakage in the DNA. This breakage leads to the inhibition of nucleic acid synthesis and therefore causes death of the bacterial or protozoal cell. -
Pharmacokinetics
Metronidazole is a moderately lipophilic, low molecular weight, weak base that penetrates cell membranes and is well absorbed systemically.
The pharmacokinetics of AYRADIA oral suspension were evaluated in a cross-over study in 6 male and 6 female Beagle dogs receiving a single oral dose of 25 mg/kg metronidazole in the fed or fasted state. Following overnight fasting, half the dogs were fed a meal of dry dog food 15 minutes before dosing and the other dogs continued to be fasted until 4 hours post metronidazole administration. Blood samples were collected prior to dosing and at 0.25, 0.5, 1, 1.5, 2, 4, 6, 8, 12, 24, 30, and 48 hours post- dosing. Plasma concentrations of metronidazole were measured using a liquid chromatography with mass spectrometry detection method.
Table 1. Mean (Standard Deviation) Pharmacokinetic Parameters for Plasma Metronidazole.
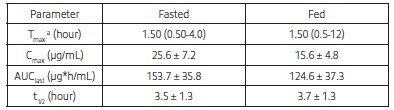
aMedian and Range
Tmax = time to maximum plasma concentration
Cmax = maximum plasma concentration
AUClast = area under the curve from the time of dosing to the last quantifiable plasma concentration
t1/2 = elimination half-lifeThe maximum plasma concentration (Cmax) and area under the curve from the time of dosing to the last quantifiable plasma concentration (AUClast) for metronidazole were 39 and 20% lower, respectively, in the fed state, as compared to the fasted state. In a separate cross-over study, following oral and intravenous administration of 25 mg/kg metronidazole to twelve Beagle dogs, the mean absolute bioavailability, clearance (CL), and volume of distribution (Vd) of metronidazole oral suspension were 97.6%,
118 mL/hour/kg, and 628 mL/kg, respectively. -
SUMMARY OF SAFETY AND EFFECTIVENESS
Effectiveness
The effectiveness of AYRADIA oral suspension was demonstrated in one dose confirmation laboratory study and one field effectiveness and safety study. AYRADIA oral suspension was administered at a dose of 25 mg/kg twice daily for five consecutive days in both studies.The dose confirmation laboratory study was a parallel, masked, negative (sterile water) controlled study to evaluate effectiveness in naturally occurring Giardia duodenalis infection in dogs based on post-treatment intestinal trophozoite counts. The study included 13 healthy beagle dogs naturally infected with Giardia with pre-treatment cyst counts of ≥ 750 cysts/gram feces. The dogs were between 8.1 and 10.9 months of age and weighed between 9.8 and 14.6 kg (21.6 and 32.2 lbs). A statistically significant difference in trophozoite counts on Day 8, four days after the last dose, was detected in the AYRADIA-treated group as compared to the control group (geometric mean counts in the control group = 339,617 versus 1,056 in the treated group; p = 0.0026). The relative difference between the AYRADIA-treated group and the control group was calculated to be 99.7%.
The field effectiveness and safety study was a double-masked, vehicle-controlled, randomized, multi-center study conducted at veterinary clinics and shelters or non-breed-specific rescue groups to evaluate the effectiveness of AYRADIA oral suspension to treat dogs naturally infected with Giardia duodenalis. Effectiveness was evaluated in 120 of the 180 dogs enrolled (80 in the AYRADIA oral suspension group and 40 in the control group). Dogs ranged in age from 5 weeks to 15.2 years old, weighed 2.0 and 37.2 kg (4.4 and 81.8 lbs), were of various pure or mixed breeds, and included intact, non-pregnant and non-lactating, or spayed females, and intact or neutered males.
Observations included baseline physical examination, body weight, hematology, serum chemistry, and urinalysis before and after treatment. In addition, three daily fecal samples before and three daily fecal after treatment were obtained for immunofluorescence assay (IFA) cyst counts. Safety was monitored during the study by documentation of adverse events (see Adverse Reactions).
The difference between AYRADIA oral suspension and the vehicle control in terms of post-treatment cyst counts was significantly different (p<0.001) and in favor of the AYRADIA-treated dogs. Additionally, there was a 99.9% percent reduction in cyst counts between baseline and post-treatment in the AYRADIA-treated group. Based on these results, AYRADIA oral suspension was demonstrated to be effective for the treatment of Giardia duodenalis in naturally infected dogs.
Target Animal Safety
In a laboratory safety study, 12-week old, healthy, Beagle puppies (4/sex/group) were administered AYRADIA oral suspension at 0X, 1X, 2X and 3X the therapeutic dose (25 mg/kg twice per day) for 15 days (3X the treatment duration) and at 5X the therapeutic dose for 5 days (the treatment duration). AYRADIA oral suspension at 5X the therapeutic dose was associated with self-limiting episodes of diarrhea and erythema of the ears. No other clinically relevant observations were noted during the study.In a four-dog laboratory tolerance study, two dogs, approximately four months old, received an investigational metronidazole oral suspension formulation (160 mg/mL) at 500 mg/kg/day (10X the intended daily dose) for 7 days, and two dogs (one 12 months old and one 21 months old) received a commercially-available metronidazole tablet at 250 mg/kg/day (5X the intended daily dose) for 7 days. No abnormal clinical signs were observed in dogs treated at 250 mg/kg/day with the metronidazole tablet. The two dogs that received the metronidazole oral suspension at 500 mg/kg/day exhibited severe neurologic signs by Days 7 and 8, respectively. Ataxia and lack of ocular reflexes were observed in both dogs. Additional adverse signs in one of the dogs included lateral movements of head and eyes, recumbency, tremors, and mydriasis. The dogs recovered after cessation of metronidazole administration and treatment with repeated doses of diazepam and furosemide. Both dogs recovered fully within approximately 24 hours.
- Storage Conditions
- How Supplied
- SPL UNCLASSIFIED SECTION
- Instructions for Using the Dispensing System and Preparing a Dose of AYRADIA oral suspension.
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
AYRADIA
metronidazole solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 51311-034 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METRONIDAZOLE (UNII: 140QMO216E) (METRONIDAZOLE - UNII:140QMO216E) METRONIDAZOLE 125 mg in 1 mL Product Characteristics Color brown Score Shape Size Flavor LIVER Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51311-034-30 1 in 1 CARTON 1 30 mL in 1 BOTTLE 2 NDC: 51311-034-10 1 in 1 CARTON 2 100 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141572 12/11/2023 Labeler - Virbac AH Inc (131568396) Registrant - Virbac AH Inc (131568396)
Trademark Results [AYRADIA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 AYRADIA 97480979 not registered Live/Pending |
Virbac 2022-06-29 |
 AYRADIA 88266830 not registered Live/Pending |
Virbac 2019-01-18 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.




