FLUBLOK- influenza vaccine injection, solution
Flublok by
Drug Labeling and Warnings
Flublok by is a Other medication manufactured, distributed, or labeled by Protein Sciences Corporation, Hospira, Inc., MassBiologics. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use Flublok® safely and effectively. See full prescribing information for Flublok.
Flublok (Influenza Vaccine)
Sterile Solution for Intramuscular Injection
2016-2017 Formula
Initial U.S. Approval: 2013RECENT MAJOR CHANGES
Indications and Usage (1) 10/2014
INDICATIONS AND USAGE
- Flublok is a vaccine indicated for active immunization against disease caused by influenza virus subtypes A and type B contained in the vaccine. Flublok is approved for use in persons 18 years of age and older. (1)
- In persons 18 through 49 years of age, this indication is based on a controlled clinical study demonstrating a decrease in influenza disease after vaccination with Flublok. In persons 50 years of age and older, this indication is based on the immune response elicited by Flublok; data demonstrating a decrease in influenza disease in persons 50 years and older after vaccination with Flublok are not available. (14)
DOSAGE AND ADMINISTRATION
- For intramuscular (IM) injection only (0.5 mL). (2)
DOSAGE FORMS AND STRENGTHS
A sterile solution for injection supplied in 0.5mL single dose vials. (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Appropriate medical treatment and supervision must be available to manage possible anaphylactic reactions following administration of Flublok. (5.1)
- If Guillain-Barré syndrome has occurred within 6 weeks of receipt of a prior influenza vaccine, the decision to give Flublok should be based on careful consideration of potential benefits and risks. (5.2)
ADVERSE REACTIONS
- In adults 18 through 49 years of age, the most common (≥10%) injection-site reaction was pain (37%); the most common (≥10%) solicited systemic adverse reactions were headache (15%), fatigue (15%) and myalgia (11%). (6.1)
- In adults 50 through 64 years of age, the most common (≥10%) injection site reaction was pain (32%); the most common (≥10%) solicited systemic adverse reactions were headache (17%), fatigue (13%), and muscle pain (11%). (6.1)
- In adults 65 years of age and older, the most common (≥10%) injection site reaction was pain (19%); the most common (≥10%) solicited systemic adverse reactions were fatigue (13%) and headache (10%). (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Protein Sciences Corporation at 1-888-855-7871 or VAERS at 1-800-822-7967 or www.vaers.hhs.gov.
USE IN SPECIFIC POPULATIONS
- Pregnancy: A registry is available for Flublok. Contact: Protein Sciences Corporation by calling 1-888-855-7871. (8.1)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 7/2016
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosage
2.2 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Managing Allergic Reactions
5.2 Guillain Barré Syndrome
5.3 Altered Immunocompetence
5.4 Limitations of Vaccine Effectiveness
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
13 NONCLINICAL TOXICOLOGY
14 CLINICAL STUDIES
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
Flublok is a vaccine indicated for active immunization against disease caused by influenza virus subtypes A and type B contained in the vaccine. Flublok is approved for use in persons 18 years of age and older.
In persons 18 through 49 years of age, this indication is based on a controlled clinical study demonstrating a decrease in influenza disease after vaccination with Flublok. In persons 50 years of age and older, this indication is based on the immune response elicited by Flublok; data demonstrating a decrease in influenza disease in persons 50 years and older after vaccination with Flublok are not available. (see Clinical Studies[14])
-
2 DOSAGE AND ADMINISTRATION
For intramuscular injection only.
2.2 Administration
Shake the single-dose vial gently before withdrawing the vaccine dose.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permits. If either of these conditions exists, the vaccine should not be administered.
The preferred site for injection is the deltoid muscle. Administration is by sterile needle and syringe.
Flublok should not be mixed with any other vaccine in the same syringe or vial.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Managing Allergic Reactions
Appropriate medical treatment and supervision must be available to manage possible anaphylactic reactions following administration of the vaccine.
5.2 Guillain Barré Syndrome
The 1976 swine influenza vaccine was associated with an increased frequency of Guillain-Barré Syndrome (GBS). Evidence for a causal relation of GBS with other influenza vaccines is inconclusive; if an excess risk exists, it is probably slightly more than one additional case per 1 million persons vaccinated. If GBS has occurred within 6 weeks of receipt of a prior influenza vaccine, the decision to give Flublok should be based on careful consideration of the potential benefits and risks.
-
6 ADVERSE REACTIONS
In adults 18 through 49 years of age, the most common (≥10%) injection-site reaction was pain (37%); the most common (≥10%) solicited systemic adverse reactions were headache (15%), fatigue (15%) and muscle pain (11%). (6.1)
In adults 50 through 64 years of age, the most common (≥10%) injection site reaction was pain (32%); the most common (≥10%) solicited systemic adverse reactions were headache (17%), fatigue (13%), and muscle pain (11%). (6.1)
In adults 65 years of age and older, the most common (≥10%) injection site reaction was pain (19%); the most common (≥10%) solicited systemic adverse reactions were fatigue (13%) and headache (10%). (6.1)
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a vaccine cannot be directly compared to rates in the clinical studies of another vaccine and may not reflect the rates observed in clinical practice.
Flublok has been administered to and safety data collected from 2497 adults 18 through 49 years of age, 972 adults 50 through 64 years of age, and 1078 adults aged 65 years and older enrolled in five randomized, placebo- or active-controlled clinical trials. Clinical safety data for Flublok are presented from four clinical trials (Studies 1, 2, 3, and 4). Data from a placebo-controlled trial in adults 18 through 49 years of age (Study 1) are presented, followed by data pooled according to age group from Studies 2 and 4 (adults 50 through 64 years of age) and Studies 3 and 4 (adults aged 65 years and older). Reactogenicity data from a small Phase 2 trial (Study 5) in adults 18 through 49 years of age, 153 of whom received Flublok 135mcg, are not presented. However, subjects from Study 5 are included in the description of deaths and serious adverse events (SAEs). In all studies local (injection site) and systemic adverse reactions were solicited with the use of a memory aid for 7 days following vaccination, and unsolicited adverse reactions were collected for 28-30 days post-vaccination. In Studies 1- 3 and 5, SAEs were collected for 6 months post-vaccination via clinic visit or telephone follow up on Day 28, telephone follow up on Day 180, or by spontaneous reporting. Study 4 collected SAEs through 30 days following receipt of vaccine. Study 4 also actively solicited pre-specified common hypersensitivity-type reactions through 30 days following receipt of vaccine as a primary endpoint.
Study 1 included 4648 subjects 18 through 49 years of age for safety analysis, randomized to receive Flublok (n=2344) or placebo (n=2304) (2) (see Clinical Studies [14]).
Study 2 included 602 subjects 50 through 64 years of age for safety analysis, randomized to receive Flublok (n=300) or another U.S.-licensed trivalent influenza vaccine (Fluzone, manufactured by Sanofi Pasteur, Inc.) as an active control (n=302) (3) (see Clinical Studies [14]).
Study 3 included 869 subjects aged 65 years and older for safety analysis, randomized to receive Flublok (n=436) or another U.S.-licensed trivalent influenza vaccine (Fluzone) as an active control (n=433) (4) (see Clinical Studies [14]).
Study 4 included 2627 subjects aged 50 years and older for safety analysis, randomized to receive Flublok (n=1314) or another U.S.-licensed trivalent influenza vaccine (Afluria, manufactured by bioCSL Pty Ltd.) as an active control (n=1313). Among subjects 50 through 64 years of age, 672 received Flublok and 665 received Afluria. Among subjects aged 65 years and older, 642 received Flublok and 648 received Afluria (see Clinical Studies [14]).
In a clinical trial of adults 18-49 years of age (Study 1, Table 1) the mean age of participants was 32.5 years, 59% were female, and 67% were Caucasian (see Clinical Studies [14]).
Table 1: Frequency of Solicited Local Injection Site Reactions and Systemic Adverse Reactions within 7 Days of Administration of Flublok or Placebo in Adults 18-49 Years of Age, Study 1, Total Vaccinated Cohort1,2,3 Flublok
N=2272Placebo
N=2231Local % % Any Mod4 Sev4 Any Mod4 Sev4 Pain
37
2
<1
8
<1
<1
Redness
4
<1
<1
2
<1
<1
Swelling
3
<1
<1
2
<1
<1
Bruising
3
<1
<1
3
<1
<1
Systemic
%
%
Headache
15
3
<1
16
3
<1
Fatigue
15
3
<1
14
3
<1
Muscle Pain
11
2
<1
7
<1
<1
Nausea
6
1
<1
5
1
<1
Joint pain
4
<1
<1
4
<1
<1
Chills
3
<1
<1
3
<1
<1
Fever‡
<1
<1
<1
<1
<1
<1
- NOTE: Data based on the most severe response reported by subjects. Results ≥1% reported to nearest whole percent; results >0 but <1% reported as <1%.
‡ Fever defined as ≥100.4°F (38°C). Mild (≥100.4º to <101.1ºF); Moderate (≥101.2ºF to <102.2ºF); Severe (≥102.2ºF)
1 Total Vaccinated Cohort is defined as all randomized subjects who received study vaccine according to the treatment actually received and who provided data.
2 Study 1 is registered as NCT00539981 under the National Clinical Trials registry.
3 Denominators for Study 1: The total number of enrolled, randomized, and vaccinated subjects was 2344 in the Flublok group and 2304 in the placebo group. For all categories except fever, the number of subjects with missing values was 72 in the Flublok group and 73 in the Placebo group so that these denominators are 2272 and 2231 respectively. For fever, 89 Flublok recipients and 104 Placebo recipients were missing data, making these denominators 2255 and 2200 respectively.
4 Moderate = had it, and it was bad enough to prevent a significant part of usual activities; Severe = had it, and it prevented most or all of normal activities, or had to see a doctor for prescription medicine.
Across three clinical trials (Studies 2 – 4, Tables 2 and 3) a total of 2050 adults age 50 years and older received Flublok and 2048 received a U.S.-licensed IIV3 comparator. The mean age of these study participants was 65 years; 56% were female and 80% were Caucasian (see Clinical Studies [14]).
The incidence of solicited reactogenicity differed between adults 50 through 64 years of age and adults aged 65 years and older. Therefore, data from Studies 2, 3, and 4 were pooled according to age group and are presented separately (Tables 2 and 3).
Most events in both age groups were mild in severity.
Table 2: Frequency of Solicited Local Injection Site Reactions and Systemic Adverse Reactions within 7 Days of Administration of Flublok or Comparator in Adults 50-64 Years of Age, Studies 2 and 4, Total Vaccinated Cohort1,2 Flublok
N=972IIV32
N=967Any Mod3 Sev3 Any Mod3 Sev3 Local
%
Pain
32
2
<1
37
<1
0
Firmness/Swelling
7
2
<1
6
1
<1
Redness
6
2
<1
5
1
<1
Systemic
%
Headache
17
4
<1
16
3
<1
Fatigue
13
3
<1
17
3
<1
Muscle Pain
11
2
<1
11
2
<1
Joint Pain
8
2
<1
8
2
<1
Nausea
6
1
0
5
<1
<1
Shivers/Chills
5
1
0
4
<1
<1
Fever‡
<1
<1
<1
<1
0
0
- NOTE: Data based on the most severe response reported by subjects. Results ≥1% reported to nearest whole percent; results >0 but <1% reported as <1%.
‡ Fever defined as ≥100.4°F (38°C). Mild (≥100.4º to <101.1ºF); Moderate (≥101.2ºF to <102.2ºF); Severe (≥102.2ºF) For fever, 12 Flublok recipients and 5 IIV3 recipients were missing data, making these denominators 964 and 962, respectively.
1 Total Vaccinated Cohort is defined as all randomized subjects who received study vaccine according to the treatment actually received and who provided data.
2 Pooled Data from Studies 2 and 4. For Studies 2 and 4, the U.S.-licensed IIV3 comparators were Fluzone and Afluria, respectively. Studies 2 and 4 are registered as NCT00539864 and NCT01825200, respectively, under the National Clinical Trials registry.
3 Moderate = had it, and it was bad enough to prevent a significant part of usual activities; Severe = had it, and it prevented most or all of normal activities, or had to see a doctor for prescription medicine.
Table 3: Frequency of Solicited Local Injection Site Reactions and Systemic Adverse Reactions within 7 Days of Administration of Flublok or Comparator in Adults ≥65 Years of Age, Studies 3 and 4, Total Vaccinated Cohort1,2 Flublok
N=1078IIV32
N=1081Any
Mod3
Sev3
Any
Mod3
Sev3
Local
%
Pain
19
<1
<1
20
<1
<1
Redness
7
1
<1
7
1
1
Firmness/Swelling
7
2
<1
7
<1
<1
Systemic
%
Fatigue
13
3
<1
15
2
<1
Headache
10
<1
<1
9
1
<1
Muscle Pain
8
2
<1
8
1
<1
Joint Pain
6
1
<1
6
1
<1
Shivers/Chills
5
<1
<1
5
<1
<1
Nausea
4
<1
<1
3
<1
<1
Fever‡
3
<1
<1
2
0
0
- NOTE: Data based on the most severe response reported by subjects. Results ≥1% reported to nearest whole percent; results >0 but <1% reported as <1%.
‡ Fever defined as ≥100.4°F (38°C). Mild (≥100.4º to <101.1ºF); Moderate (≥101.2ºF to <102.2ºF); Severe (≥102.2ºF)
1 Total Vaccinated Cohort is defined as all randomized subjects who received study vaccine according to the treatment actually received and who provided data.
2 Pooled Data from Studies 3 and 4. For Studies 3 and 4, the U.S.-licensed IIV3 comparators were Fluzone and Afluria, respectively. Studies 3 and 4 are registered as NCT00395174 and NCT01825200, respectively, under the National Clinical Trials registry.
3 Moderate = had it, and it was bad enough to prevent a significant part of usual activities; Severe = had it, and it prevented most or all of normal activities, or had to see a doctor for prescription medicine.
Among adults 18-49 years of age (Studies 1 and 5 pooled), through 6 months post-vaccination, two deaths were reported, one in a Flublok recipient and one in a placebo recipient. Both deaths occurred more than 28 days following vaccination and neither was considered vaccine-related. SAEs were reported by 32 Flublok recipients and 35 placebo recipients. One SAE in a Flublok recipient was assessed as possibly related to the vaccine: pleuropericarditis with effusions requiring hospitalization and drainage. No specific cause was identified. The patient recovered.
Among adults 50-64 years of age (Studies 2 and 4 pooled), through up to 6 months post-vaccination, there were no deaths; SAEs were reported by 10 subjects, 6 Flublok recipients and 4 IIV3 recipients. One of the SAEs, vasovagal syncope following injection of Flublok, was considered related to study vaccine. Among adults 65 years of age and older (Studies 3 and 4 pooled), through up to 6 months post-vaccination, there were 4 deaths, 2 in Flublok recipients and 2 in IIV3 recipients. None were considered related to the study vaccines. SAEs were reported from 80 subjects, 37 Flublok recipients, 43 in IIV3 recipients. None were considered related to the study vaccines.
In Study 1 (adults 18-49 years of age), the most frequent unsolicited adverse events, occurring in 1%-2% of subjects, were nasopharyngitis, upper respiratory infection, headache, cough, nasal congestion, pharyngolaryngeal pain, and rhinorrhea.
Among adults 50-64 years of age (Studies 2 and 4 pooled), the most frequent unsolicited adverse events, occurring in 1% of subjects, were diarrhea and cough. Among adults ≥65 years of age (Studies 3 and 4 pooled), the most frequent unsolicited adverse events, occurring in 1% of subjects, were nasopharyngitis and cough.
Among adults 50 years of age and older (Study 4) for whom the incidence of rash, urticaria, swelling, non-pitting edema, or other potential hypersensitivity reactions were actively solicited for 30 days following vaccination, a total of 2.4% of Flublok recipients and 1.6% of IIV3 recipients reported such events over the 30 day follow-up period. A total of 1.9% and 0.9% of Flublok and IIV3 recipients, respectively, reported these events in the 7 days following vaccination. Of these solicited events, rash was most frequently reported (Flublok 1.3%, IIV3 0.8%) over the 30 day follow-up period.
6.2 Postmarketing Experience
The following events have been spontaneously reported during post approval use of Flublok. They are described because of the strength of the causal relationship to Flublok and their potential seriousness. Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to vaccine exposure.
Immune system disorders: anaphylaxis, anaphylactoid reactions, allergic reactions, and other forms of hypersensitivity.
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category B
Reproduction studies have been performed in rats at a dose approximately 300 times the human dose (on a mg/kg basis) and have revealed no evidence of impaired fertility or harm to the fetus due to Flublok. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this vaccine should be used during pregnancy only if clearly needed. The effect of Flublok on embryo-fetal and pre-weaning development was evaluated in pregnant rats. Animals were administered Flublok by intramuscular injection twice prior to gestation and once during the period of organogenesis (gestation days 6), 0.5 ml/rat/occasion (approximately 300-fold excess relative to the projected human dose on a mg/kg basis). No adverse effects on mating, female fertility, pregnancy, parturition, lactation, embryo-fetal and pre-weaning development were observed. There were no vaccine-related fetal malformations or other evidence of teratogenesis.
8.3 Nursing Mothers
Flublok has not been evaluated in nursing mothers. It is not known whether Flublok is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Flublok is administered to a nursing woman.
8.4 Pediatric Use
Data from a randomized, controlled trial demonstrated that children 6 months to less than 3 years of age had diminished hemagglutinin inhibition (HAI) responses to Flublok as compared to a U.S.-licensed influenza vaccine approved for use in this population, strongly suggesting that Flublok would not be effective in children younger than 3 years of age. Safety and effectiveness of Flublok in children 3 years to less than 18 years of age have not been established.
8.5 Geriatric Use
In clinical studies, Flublok has been administered to, and safety information collected for, 1078 subjects ages 65 years and older (see Clinical Trials Experience [6.1]). Clinical effectiveness in adults aged 65 and older is based on the immune response elicited by Flublok and not on demonstration of decreased influenza disease. After administration of Flublok, hemagglutination-inhibiting antibody responses in persons 65 years of age and older were lower as compared to younger adult subjects (see Clinical Studies [14]).
-
11 DESCRIPTION
Flublok [Influenza Vaccine] is a sterile, clear, colorless solution of recombinant hemagglutinin (HA) proteins from three influenza viruses for intramuscular injection. It contains purified HA proteins produced in a continuous insect cell line (expresSF+®) that is derived from Sf9 cells of the fall armyworm, Spodoptera frugiperda (which is related to moths, caterpillars and butterflies), and grown in serum-free medium composed of chemically-defined lipids, vitamins, amino acids, and mineral salts. Each of the three HAs is expressed in this cell line using a baculovirus vector (Autographa californica nuclear polyhedrosis virus), extracted from the cells with Triton X-100 and further purified by column chromatography. The purified HAs are then blended and filled into single-dose vials.
Flublok is standardized according to United States Public Health Service (USPHS) requirements. For the 2016-2017 influenza season it is formulated to contain 135 mcg HA per 0.5 mL dose, with 45 mcg HA of each of the following 3 influenza virus strains: A/California/7/2009 (H1N1), A/Hong Kong/4801/2014 (H3N2), and B/Brisbane/60/2008.
A single 0.5 mL dose of Flublok contains sodium chloride (4.4 mg), monobasic sodium phosphate (0.195 mcg), dibasic sodium phosphate (1.3 mg), and polysorbate 20 (Tween®20) (27.5 mcg). Each 0.5 mL dose of Flublok may also contain residual amounts of baculovirus and Spodoptera frugiperda cell proteins (≤ 28.5 mcg), baculovirus and cellular DNA (≤ 10 ng), and Triton X-100 (≤ 100 mcg).
Flublok contains no egg proteins, antibiotics, or preservatives. The stoppers used for the single-dose vials are not made with natural rubber latex.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Flublok contains recombinant HA proteins of the three strains of influenza virus specified by health authorities for inclusion in the annual seasonal vaccine. These proteins function as antigens which induce a humoral immune response, measured by hemagglutination inhibition (HI) antibody.
Antibodies against one influenza virus type or subtype confer limited or no protection against another. Furthermore, antibodies to one antigenic variant of influenza virus might not protect against a new antigenic variant of the same type or subtype. Frequent development of antigenic variants through antigenic drift is the virologic basis for seasonal epidemics and the reason for the usual replacement of one or more influenza virus strains in each year's influenza vaccine. Therefore, influenza vaccines are standardized to contain the hemagglutinins of influenza virus strains (i.e., typically two type A and one type B), representing the influenza viruses likely to be circulating in the U.S. in the upcoming winter.
-
13 NONCLINICAL TOXICOLOGY
Flublok has not been evaluated for carcinogenic or mutagenic potential, or for impairment of male fertility in animals.
Reproduction studies performed in female rats revealed no evidence of impaired fertility due to Flublok (see Pregnancy [8.1]).
-
14 CLINICAL STUDIES
Efficacy Against Culture-Confirmed Influenza
The efficacy of Flublok was evaluated in a randomized, observer-blind, placebo-controlled multicenter trial conducted in the U.S. during the 2007-2008 influenza season (Study 1) (2). The trial enrolled and vaccinated 4648 healthy adults (mean age 32.5 years) randomized in a 1:1 ratio to receive a single dose of Flublok (n=2344) or saline placebo (n=2304). Among enrolled subjects, 59% were female, 67% were white, 19% African-American, 11% Latino/Hispanic, 2% Asian and < 1% other. The two groups were similar in demographics. Culture-confirmed influenza was assessed by active and passive surveillance for influenza-like illness (ILI) beginning 2 weeks post-vaccination until the end of the influenza season, approximately 7 months post- vaccination. ILI was defined as having at least 2 of 3 symptoms (no specified duration) in the following categories: 1) fever ≥ 100ºF; 2) respiratory symptoms (cough, sore throat, runny nose/stuffy nose); or 3) systemic symptoms (myalgias, arthralgias, headache, chills/sweats, tiredness/malaise). For subjects with an episode of ILI, nasal and throat swab samples were collected for viral culture.
The primary efficacy endpoint was Centers for Disease Control-defined influenza-like illness (CDC-ILI) with a positive culture for an influenza virus strain antigenically resembling a strain represented in Flublok. CDC-ILI is defined as fever of ≥100°F oral accompanied by cough, sore throat, or both on the same day or on consecutive days. Attack rates and vaccine efficacy (VE), defined as the relative reduction in the influenza rate for Flublok relative to placebo, were calculated for the total vaccinated cohort (n=4,648). The pre-defined success criterion for the primary efficacy analysis was that the lower bound of the 95% confidence interval (CI) of VE should be at least 40%. Vaccine efficacy against antigenically matched culture-confirmed CDC-ILI could not be determined reliably because 96% of the influenza isolates obtained from subjects in Study 1 were not antigenically matched to the strains represented in the vaccine. An exploratory analysis of VE of Flublok against all strains regardless of antigenic match isolated from any subject with an ILI, not necessarily CDC-defined ILI, demonstrated an efficacy estimate of 44.8% (95% CI 24.4, 60.0). See Table 4 for a presentation of VE by case definition and antigenic similarity.
Table 4: Vaccine Efficacy Against Culture-Confirmed Influenza in Healthy Adults 18-49 Years of Age, Study 1* Case definition
Flublok
(N=2344)Saline Placebo
(N=2304)Flublok
Vaccine
Efficacy1, %95%
Confidence IntervalCases, n
Rate, %
Cases, n
Rate, %
Positive culture with a strain represented in the vaccine
CDC-ILI, all matched strains2,3
1
0.04
4
0.2
75.4
(-148.0, 99.5)
Any ILI, all matched strains4,5
2
0.1
6
0.3
67.2
(-83.2, 96.8)
Positive culture with any strain, regardless of match to the vaccine
CDC-ILI, all strains2,6
44
1.9
78
3.4
44.6
(18.8, 62.6)
Sub-Type A
26
1.1
56
2.4
54.4
(26.1, 72.5)
Type B
18
0.8
23
1.0
23.1
(-49.0, 60.9)
Any ILI, all strains4
64
2.7
114
4.9
44.8
(24.4, 60.0)
Sub-Type A
41
1.7
79
3.4
49.0
(24.7, 65.9)
Type B
23
1.0
36
1.6
37.2
(-8.9, 64.5)
*In Study 1 (NCT00539981) vaccine efficacy analyses were conducted on the Total Vaccinated Cohort (all randomized subjects who received study vaccine according to the treatment actually received and who provided data). Vaccine efficacy (VE) = 1 minus the ratio of Flublok/placebo infection rates.
1 Determined under the assumption of Poisson event rates, according to Breslow and Day, 1987.
2 Meets CDC influenza-like illness (CDC-ILI) defined as fever of ≥100ºF oral accompanied by cough and/or sore throat, on the same day or on consecutive days.
3 Primary endpoint of trial.
4 All culture-confirmed cases are considered, regardless of whether they qualified as CDC-ILI.
5 Secondary endpoint of trial.
6 Exploratory (prespecified) endpoint of trial.Immunogenicity in Adults 50 years of Age and Older
Two randomized controlled clinical trials of Flublok in adults aged 50 years and older evaluated immune responses by measuring hemagglutination inhibition (HI) antibody titers to each virus strain in the vaccine in adults as compared to another U.S.-licensed trivalent influenza vaccine (IIV3). In these studies, post-vaccination immunogenicity was evaluated on sera obtained 28 days after administration of a single dose of Flublok or comparator vaccine. Hemagglutination inhibition geometric mean titers (GMTs) were determined for each vaccine antigen. Immunogenicity was evaluated by calculating GMT ratios of IIV3 to Flublok.
Study 2 was a randomized, observer-blind, comparator-controlled, multi-center trial in healthy adults 50 through 64 years of age to evaluate the immunogenicity of Flublok as compared to a U.S.-licensed IIV3 (Fluzone). (3) Results are presented in Table 5. A total of 602 subjects were enrolled, randomized 1:1, and vaccinated with Flublok (300 subjects) or IIV3 (302 subjects). Of the total vaccinated population, 601 subjects (299 Flublok and 302 IIV3 recipients, respectively) were evaluable for immune response (per protocol population for immunogenicity). Subjects were predominantly white (71%) and female (63%) with a mean age of 55.8 years. Immune response endpoints were HI GMTs for each vaccine antigen at baseline and at 28 days post-vaccination. GMTs were compared based on the upper bound of the two-sided 95% confidence interval (CI) of the GMT ratio of IIV3 to Flublok. Success in meeting this endpoint was pre-defined as an upper bound (UB) of the two-sided 95% CI of GMTIIV3 / GMTFlublok ≤ 1.5 (5). Flublok met the success criterion for HI GMTs for all three antigens. Sub-population analyses of immunogenicity did not reveal significant differences between genders. Sub-analyses according to race and ethnicity were not informative because the study population was not sufficiently diverse.
Study 3 was a randomized, observer-blind, comparator-controlled, multi-center trial in 869 medically stable elderly adults ≥65 years of age to evaluate the immunogenicity of Flublok as compared to a U.S.-licensed IIV3 (Fluzone) (4). Subjects were randomized 1:1 to receive Flublok (436 vaccinated; 431 evaluable) or IIV3 (433 vaccinated; 430 evaluable). Subjects were predominantly white (98%) and female (53%) with a mean age of 73 years. Immune responses (HI GMTs and GMT ratios) were evaluated in the same manner as for Study 2. The UB of the two-sided 95% CI for the GMT ratio of IIV3 to Flublok was ≤ 1.5 for all three vaccine antigens. Sub-population analyses of immunogenicity did not reveal significant differences between genders, but were not informative with respect to race or ethnicity because the study population was not sufficiently diverse.
Table 5: Comparison of Pre- and Post-Vaccination Geometric Mean Titers (GMT) for Flublok and Fluzone, Study 2 (adults 50 through 64 years) and Study 3 (adults ≥65 years)1,2 Study Number
Antigen
Post-vaccination
GMT
Flublok
N=299Post-vaccination
GMT
Fluzone
N=302GMT Ratio
Fluzone/Flublok
[95% CI]Study 2
Age 50-64 yearsA/H1N1
181.3
139.7
0.77
(0.75, 0.79)A/H3N2
105.4
60.9
0.58
(0.53, 0.62)B
110.9
116.0
1.05
(1.01, 1.09)Study 3
Age ≥65 yearsAntigen
Post-vaccination
GMT
Flublok
N=431Post-vaccination
GMT
Fluzone
N=430GMT Ratio
Fluzone/Flublok
[95% CI]A/H1N1
176.8
148.1
0.84
(0.81, 0.86)A/H3N2
338.5
199.2
0.59
(0.57, 0.60)B
149.6
194.8
1.3
(1.26, 1.34)Abbreviations: CI, confidence interval; GMT, geometric mean titer
1The pre-defined success criterion for the GMT ratio of Fluzone to Flublok was that the upper bound of the 2-sided 95% CI of the GMT ratio, GMT Fluzone / GMT Flublok at 28 days post-vaccination, must not exceed 1.5.
2 HI titers were assayed using BEVS-derived (non-egg-derived) antigens.
-
15 REFERENCES
1. Treanor JJ, Schiff GM, Hayden FG, et.al. Safety and immunogenicity of a baculovirus-expressed hemagglutinin influenza vaccine: a randomized controlled trial. JAMA. 2007, Vol. 297, pp. 1577-1582.
2. Treanor JJ, El Sahly HM, King J, et. al. Protective efficacy of a trivalent recombinant hemagglutinin protein vaccine (FluBlok) against influenza in healthy adults: a randomized, placebo-controlled trial. Vaccine. 2011, Vol. 29, pp. 7733-7739.
3. Baxter R, Patriarca PA, Ensor K, et al. Evaluation of the safety, reactogenicity and immunogenicity of FluBlok trivalent recombinant baculovirus-expressed hemagglutinin influenza vaccine administered intramuscularly to healthy adults 50-64 years of age. Vaccine. 2011, Vol. 29, pp. 2272-2278.
4. Keitel WA, Treanor JJ, El Sahly HM, et.al. Comparative immunogenicity of recombinant influenza hemagglutinin (rHA) and trivalent inactivated vaccines (TIVs) among persons ≥65 years old. Vaccine. 2009, Vol. 28, pp. 379-385.
5. CBER/FDA. Guidance for Industry: Clinical Data Needed to Support the Licensure of Seasonal Inactivated Influenza Vaccines. s.l. : DHHS/CBER/FDA, 2007.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Flublok is supplied as a single-dose, 0.5 mL vial in a 10 vial carton:
Presentation
Carton NDC Number
Components and NDC Number
Single-Dose Vial
42874-016-10
Ten 0.5 mL single-dose vials [NDC: 42874-016-01]
-
17 PATIENT COUNSELING INFORMATION
Inform the vaccine recipient of the potential benefits and risks of vaccination with Flublok.
Inform the vaccine recipient that:
- Flublok contains non-infectious proteins that cannot cause influenza.
- Flublok stimulates the immune system to produce antibodies that help protect against influenza viruses contained in the vaccine, but does not prevent other respiratory infections.
Instruct the vaccine recipient to report any adverse events to their healthcare provider and/or to the Vaccine Adverse Event Reporting System (VAERS).
Provide the vaccine recipient with the Vaccine Information Statements which are required by the National Childhood Vaccine Injury Act of 1986 to be given prior to vaccination. These materials are available free of charge at the Centers for Disease Control (CDC) website (www.cdc.gov/vaccines).
Inform the vaccine recipient that safety and efficacy have not been established in pregnant women. Register women who receive Flublok while pregnant in the pregnancy registry by calling 1-888-855-7871.
Instruct the vaccine recipient that annual vaccination to prevent influenza is recommended.
Manufactured by Protein Sciences Corporation (Meriden, CT)
US license 1795
Distributed by Protein Sciences Corporation
Flublok is a registered trademark of Protein Sciences Corporation.
-
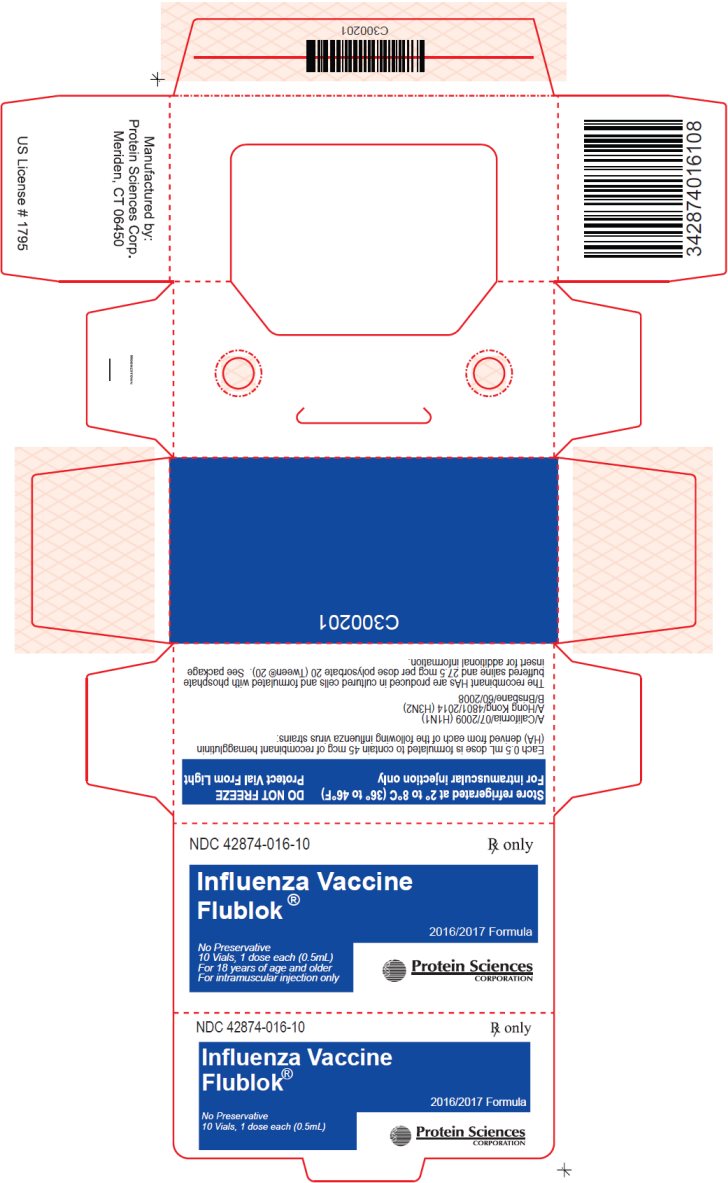
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – Carton
NDC: 42874-016-10
Rx only
Influenza Vaccine
Flublok®
2016/2017 FormulaNo Preservative
10 Vials, 1 dose each (0.5mL)
For 18 years of age and older
For intramuscular injection onlyProtein Sciences Corporation
Store refrigerated at 2° to 8°C (36° to 46°F)
For intramuscular injection onlyDO NOT FREEZE
Protect Vial From LightEach 0.5 mL dose is formulated to contain 45 mcg of recombinant hemagglutinin (HA) derived from each of the following influenza virus strains:
A/California/07/2009 (H1N1)
A/Hong Kong/4801/2014 (H3N2)
B/Brisbane/60/2008The recombinant HAs are produced in cultured cells and formulated with phosphate buffered saline and 27.5 mcg per dose polysorbate 20 (Tween® 20). See package insert for additional information.
Manufactured by:
Protein Sciences Corp.
Meriden, CT 06450US License # 1795
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - Vial
For intramuscular
use onlyNDC: 42874-016-01
Influenza Vaccine
Flublok®
2016/2017 FormulaNo Preservative
1 Dose (0.5 mL)Rx only
Protein Sciences Corp
Meriden, CT 06450
US License # 1795Sterile Single Dose
(01)00342874016016
L300101 -
INGREDIENTS AND APPEARANCE
FLUBLOK
influenza vaccine injection, solutionProduct Information Product Type VACCINE Item Code (Source) NDC: 42874-016 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength INFLUENZA A VIRUS A/CALIFORNIA/7/2009 (H1N1) RECOMBINANT HEMAGGLUTININ ANTIGEN (UNII: 1SE9Z7D2QR) (INFLUENZA A VIRUS A/CALIFORNIA/7/2009 (H1N1) RECOMBINANT HEMAGGLUTININ ANTIGEN - UNII:1SE9Z7D2QR) INFLUENZA A VIRUS A/CALIFORNIA/7/2009 (H1N1) RECOMBINANT HEMAGGLUTININ ANTIGEN 45 ug in 0.5 mL INFLUENZA A VIRUS A/HONG KONG/4801/2014 (H3N2) RECOMBINANT HEMAGGLUTININ ANTIGEN (UNII: 2I1EOL437M) (INFLUENZA A VIRUS A/HONG KONG/4801/2014 (H3N2) RECOMBINANT HEMAGGLUTININ ANTIGEN - UNII:2I1EOL437M) INFLUENZA A VIRUS A/HONG KONG/4801/2014 (H3N2) RECOMBINANT HEMAGGLUTININ ANTIGEN 45 ug in 0.5 mL INFLUENZA B VIRUS B/BRISBANE/60/2008 RECOMBINANT HEMAGGLUTININ ANTIGEN (UNII: 058U2312CR) (INFLUENZA B VIRUS B/BRISBANE/60/2008 RECOMBINANT HEMAGGLUTININ ANTIGEN - UNII:058U2312CR) INFLUENZA B VIRUS B/BRISBANE/60/2008 RECOMBINANT HEMAGGLUTININ ANTIGEN 45 ug in 0.5 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) SODIUM PHOSPHATE, DIBASIC, DODECAHYDRATE (UNII: E1W4N241FO) POLYSORBATE 20 (UNII: 7T1F30V5YH) OCTOXYNOL-9 (UNII: 7JPC6Y25QS) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 42874-016-10 10 in 1 CARTON 1 NDC: 42874-016-01 0.5 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125285 08/01/2016 Labeler - Protein Sciences Corporation (109124933) Establishment Name Address ID/FEI Business Operations Hospira, Inc. 030606222 MANUFACTURE(42874-016) , ANALYSIS(42874-016) Establishment Name Address ID/FEI Business Operations MassBiologics 038051228 MANUFACTURE(42874-016) , ANALYSIS(42874-016) Establishment Name Address ID/FEI Business Operations Protein Sciences Corporation 109124933 API MANUFACTURE(42874-016) , ANALYSIS(42874-016) Establishment Name Address ID/FEI Business Operations Protein Sciences Corporation 078739306 API MANUFACTURE(42874-016) , ANALYSIS(42874-016)
Trademark Results [Flublok]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 FLUBLOK 78415179 3088526 Live/Registered |
Protein Sciences Corporation 2004-05-07 |
 FLUBLOK 76617220 3151374 Live/Registered |
Protein Sciences Corporation 2004-10-21 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.