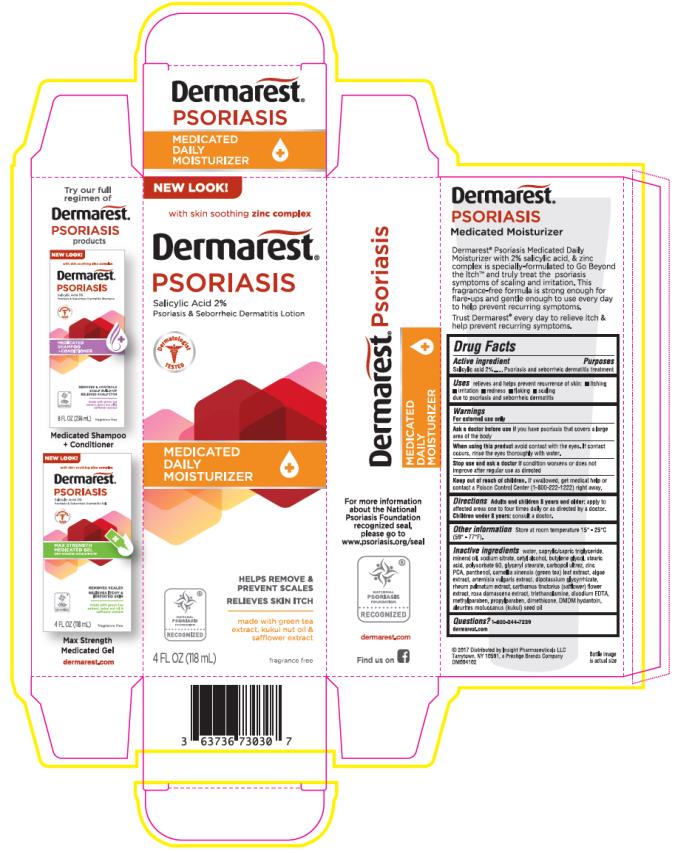
DERMAREST PSORIASIS MEDICATED MOISTURIZER- salicylic acid lotion
Dermarest by
Drug Labeling and Warnings
Dermarest by is a Otc medication manufactured, distributed, or labeled by Insight Pharmaceuticals LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purposes
- Uses
- Warnings
- Directions
- Other information
-
Inactive ingredients
Water, caprylic/capric triglyceride, mineral oil, sodium citrate, cetyl alcohol, butylene glycol, stearic acid, polysorbate 60, glyceryl stearate, carbopol ultrez, zinc PCA, panthenol, camellia sinensis (green tea) leaf extract, algae extract, artemisia vulgaris extract, dipotassium glycyrrhizate, rheum palmatum extract, carthamus tinctorius (safflower) flower extract, rosa damascena extract, triethanolamine, disodium EDTA, methylparaben, propylparaben, dimethicone, DMDM hydantoin, aleurites moluccanus (kukui) seed oil
- Questions?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DERMAREST PSORIASIS MEDICATED MOISTURIZER
salicylic acid lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 63736-073 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 2 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) MINERAL OIL (UNII: T5L8T28FGP) SODIUM CITRATE (UNII: 1Q73Q2JULR) CETYL ALCOHOL (UNII: 936JST6JCN) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) STEARIC ACID (UNII: 4ELV7Z65AP) POLYSORBATE 60 (UNII: CAL22UVI4M) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) CARBOMER INTERPOLYMER TYPE A (55000 CPS) (UNII: 59TL3WG5CO) ZINC PIDOLATE (UNII: C32PQ86DH4) PANTHENOL (UNII: WV9CM0O67Z) GREEN TEA LEAF (UNII: W2ZU1RY8B0) GLYCYRRHIZINATE DIPOTASSIUM (UNII: CA2Y0FE3FX) SAFFLOWER (UNII: 4VBL71TY4Y) ROSA DAMASCENA FLOWER OIL (UNII: 18920M3T13) TROLAMINE (UNII: 9O3K93S3TK) EDETATE DISODIUM (UNII: 7FLD91C86K) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) DIMETHICONE (UNII: 92RU3N3Y1O) DMDM HYDANTOIN (UNII: BYR0546TOW) KUKUI NUT OIL (UNII: TP11QR7B8R) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63736-073-24 24 in 1 CASE 07/23/2010 1 1 in 1 CARTON 1 118 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part358H 07/23/2010 Labeler - Insight Pharmaceuticals LLC (055665422)
Trademark Results [Dermarest]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 DERMAREST 85611679 4261139 Live/Registered |
Insight Pharmaceuticals LLC 2012-04-30 |
 DERMAREST 77564793 3626182 Live/Registered |
Insight Pharmaceuticals LLC 2008-09-08 |
 DERMAREST 73713076 1511674 Dead/Cancelled |
KINETIC SUPPORT SYSTEMS, INC. 1988-02-24 |
 DERMAREST 73706215 1499210 Dead/Cancelled |
COMMERCE DRUG CO., INC. 1988-01-19 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.