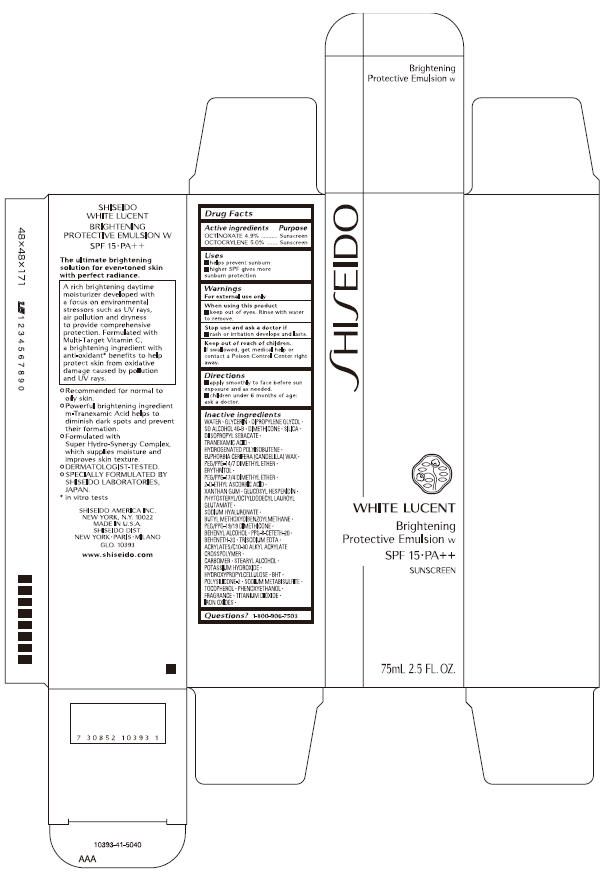
SHISEIDO WHITE LUCENT- octinoxate and octocrylene emulsion
SHISEIDO WHITE LUCENT by
Drug Labeling and Warnings
SHISEIDO WHITE LUCENT by is a Otc medication manufactured, distributed, or labeled by SHISEIDO AMERICA INC.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
- Warnings
- Directions
-
Inactive ingredients
WATER, GLYCERIN, DIPROPYLENE GLYCOL, SD ALCOHOL 40-B, DIMETHICONE, SILICA, DIISOPROPYL SEBACATE, TRANEXAMIC ACID, HYDROGENATED POLYISOBUTENE, EUPHORBIA CERIFERA (CANDELILLA) WAX, PEG/PPG-14/7 DIMETHYL ETHER, ERYTHRITOL, PEG/PPG-17/4 DIMETHYL ETHER, 2-O-ETHYL ASCORBIC ACID, XANTHAN GUM GLUCOSYL HESPERIDIN, PHYTOSTERYL/OCTYLDODECYL LAUROYL GLUTAMATE, SODIUM HYALURONATE, BUTYL METHOXYDIBENZOYLMETHANE PEG/PPG-19/19 DIMETHICONE, BEHENYL ALCOHOL, PPG-8-CETETH-20, BEHENETH-20, TRISODIUM EDTA, ACRYLATES/C10-30 ALKYL ACRYLATE CROSSPOLYMER, CARBOMER, STEARYL ALCOHOL, POTASSIUM HYDROXIDE, HYDROXYPROPYLCELLULOSE, BHT, POLYSILICONE-2, SODIUM METABISULFITE, TOCOPHEROL, PHENOXYETHANOL, FRAGRANCE, TITANIUM DIOXIDE, IRON OXIDES
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 75 mL Carton
-
INGREDIENTS AND APPEARANCE
SHISEIDO WHITE LUCENT
octinoxate and octocrylene emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 52686-228 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 3.479 g in 71 g OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 3.55 g in 71 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52686-228-10 1 in 1 CARTON 02/01/2010 1 71 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 02/01/2010 Labeler - SHISEIDO AMERICA INC. (782677132) Establishment Name Address ID/FEI Business Operations SHISEIDO AMERICA INC. 782677132 analysis(52686-228) , manufacture(52686-228)
Trademark Results [SHISEIDO WHITE LUCENT]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SHISEIDO WHITE LUCENT 76405170 3042165 Live/Registered |
Shiseido Company, Ltd. 2002-05-09 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.