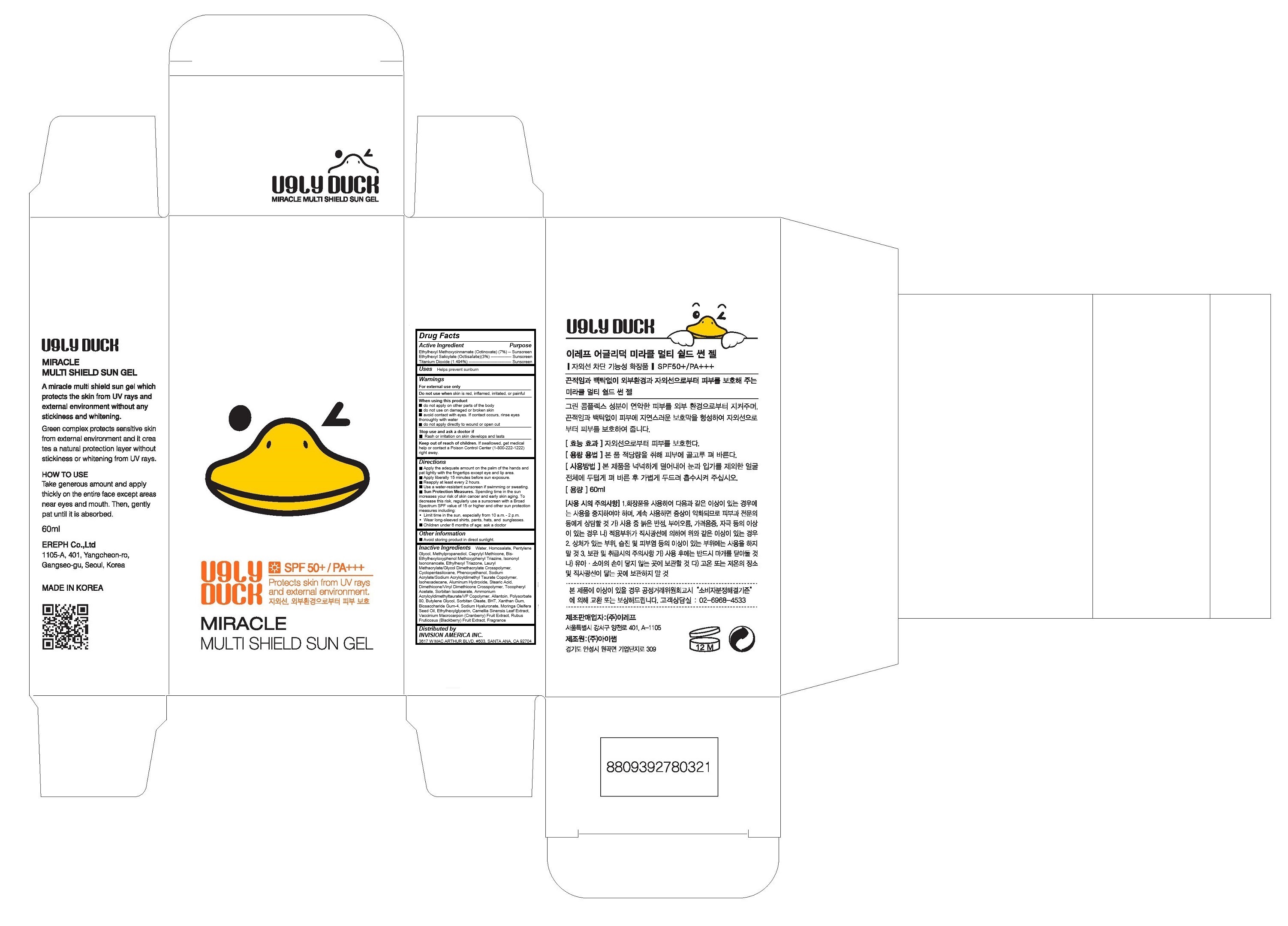
UGLY DUCK MIRACLE MULTI SHIELD SUN GEL- octinoxate, octisalate, titanium dioxide, gel
Ugly Duck Miracle Multi Shield Sun Gel by
Drug Labeling and Warnings
Ugly Duck Miracle Multi Shield Sun Gel by is a Otc medication manufactured, distributed, or labeled by EREPH CO., LTD, EYESOME. Co.,Ltd. . Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active Ingredients
- Purpose
- Keep out of reach of children
-
Warnings
For external use only
Do not use when skin is red, inflamed, irritated, or painful
When using this product
- do not apply on other parts of the body
- do not use on damaged or broken skin
- avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water
- do not apply directly to wound or open cut
Stop use and ask a doctor if
- Rash or irritation on skin develops and lasts - Uses
-
Directions
Apply liberally 15 minutes before sun exposure.
Reapply at least every 2 hours.
Use a water-resistant sunscreen if swimming or sweating.
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
Limit time in the sun, especially from 10 a.m. - 2 p.m.
Wear long-sleeved shirts, pants, hats, and sunglasses.
Children under 6 months of age: ask a doctor -
Inactive Ingredients
Water, Homosalate, Pentylene Glycol, Methylpropanediol, Caprylyl Methicone, Bis-Ethylhexyloxyphenol Methoxyphenyl Triazine, Isononyl Isononanoate, Ethylhexyl Triazone, Lauryl Methacrylate/Glycol Dimethacrylate Crosspolymer, Cyclopentasiloxane, Phenoxyethanol, Sodium Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Isohexadecane, Aluminum Hydroxide, Stearic Acid, Dimethicone/Vinyl Dimethicone Crosspolymer, Tocopheryl Acetate, Sorbitan Isostearate, Ammonium Acryloyldimethyltaurate/VP Copolymer, Allantoin, Polysorbate 80, Butylene Glycol, Sorbitan Oleate, BHT, Xanthan Gum, Biosaccharide Gum-4, Sodium Hyaluronate, Moringa Oleifera Seed Oil, Ethylhexylglycerin, Camellia Sinensis Leaf Extract, Vaccinium Macrocarpon (Cranberry) Fruit Extract,
- Ugly Duck Miracle Multi Shield Sun Gel
-
INGREDIENTS AND APPEARANCE
UGLY DUCK MIRACLE MULTI SHIELD SUN GEL
octinoxate, octisalate, titanium dioxide, gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 71624-100 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) Octinoxate 4.2 g in 60 mL Octisalate (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) Octisalate 1.8 g in 60 mL Titanium Dioxide (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) Titanium Dioxide 0.8964 g in 60 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Homosalate (UNII: V06SV4M95S) Pentylene Glycol (UNII: 50C1307PZG) Methylpropanediol (UNII: N8F53B3R4R) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) BEMOTRIZINOL (UNII: PWZ1720CBH) Isononyl Isononanoate (UNII: S4V5BS6GCX) Ethylhexyl Triazone (UNII: XQN8R9SAK4) LAURYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EX0F4CZ66H) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) Phenoxyethanol (UNII: HIE492ZZ3T) SODIUM ACRYLATE/SODIUM ACRYLOYLDIMETHYLTAURATE COPOLYMER (4000000 MW) (UNII: 1DXE3F3OZX) Isohexadecane (UNII: 918X1OUF1E) Aluminum Hydroxide (UNII: 5QB0T2IUN0) Stearic Acid (UNII: 4ELV7Z65AP) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) Allantoin (UNII: 344S277G0Z) Polysorbate 80 (UNII: 6OZP39ZG8H) Butylene Glycol (UNII: 3XUS85K0RA) SORBITAN MONOOLEATE (UNII: 06XEA2VD56) TRIS-BHT MESITYLENE (UNII: 51DM34B894) Xanthan Gum (UNII: TTV12P4NEE) BIOSACCHARIDE GUM-4 (UNII: 9XRL057X90) HYALURONATE SODIUM (UNII: YSE9PPT4TH) MORINGA OLEIFERA SEED OIL (UNII: REM6A5QMC0) Ethylhexylglycerin (UNII: 147D247K3P) GREEN TEA LEAF (UNII: W2ZU1RY8B0) CRANBERRY (UNII: 0MVO31Q3QS) Blackberry (UNII: 8A6OMU3I8L) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 71624-100-02 1 in 1 PACKAGE 07/26/2017 1 NDC: 71624-100-01 60 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 07/26/2017 Labeler - EREPH CO., LTD (690038933) Registrant - EREPH CO., LTD (690038933) Establishment Name Address ID/FEI Business Operations EREPH CO., LTD 690038933 relabel(71624-100) Establishment Name Address ID/FEI Business Operations EYESOME. Co.,Ltd. 557795360 manufacture(71624-100)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.