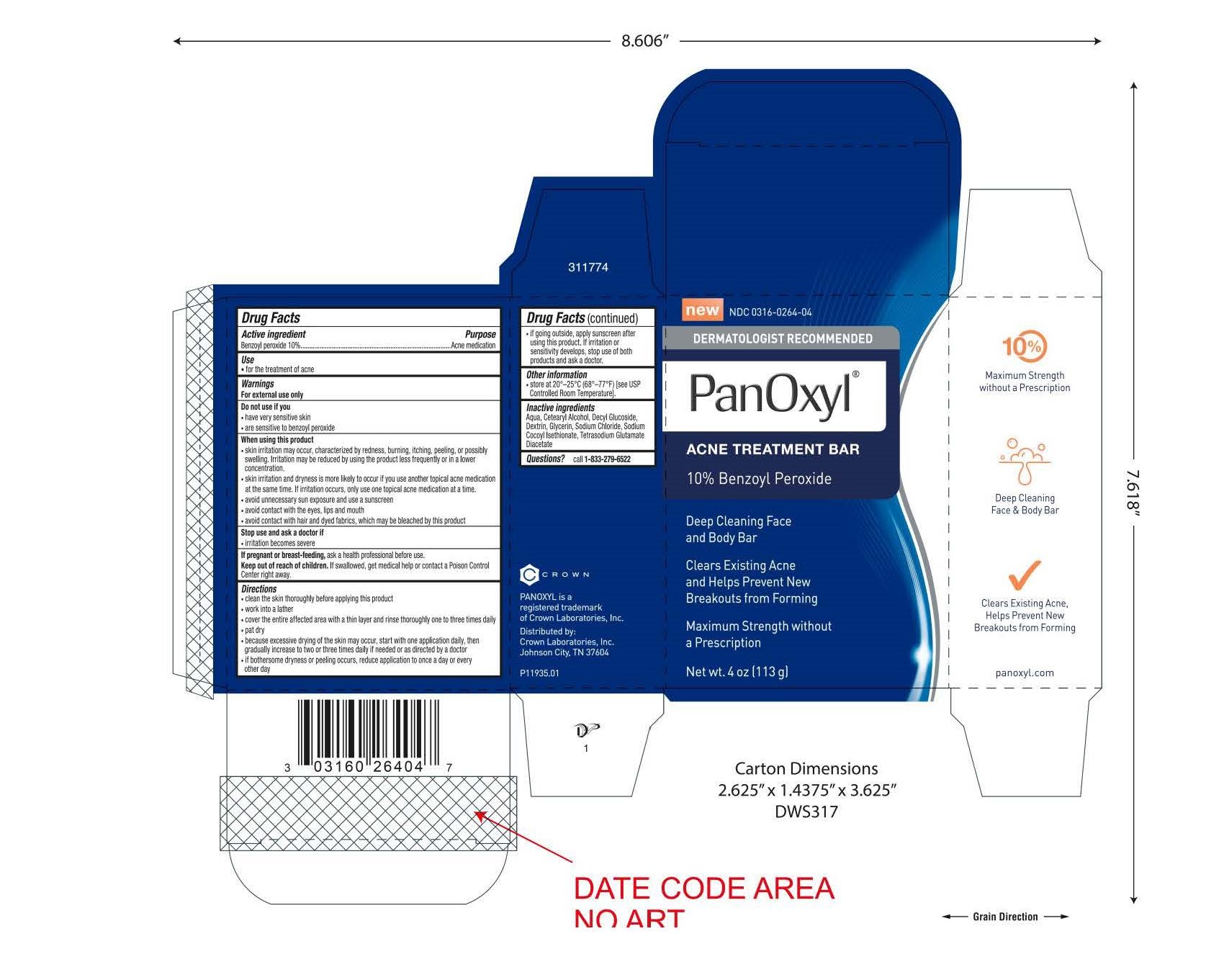
Panoxyl Acne Cleansing Bar
Panoxyl by
Drug Labeling and Warnings
Panoxyl by is a Otc medication manufactured, distributed, or labeled by The Original Bradford Soap Works Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
PANOXYL- benzoyl peroxide soap
The Original Bradford Soap Works Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Panoxyl Acne Cleansing Bar
When using this product
- skin irritation may occur, characterized by redness, burning, itching, peeling, or possibly swelling. Irritation may be reduced by using the product less frequently or in a lower concentration.
- skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
- avoid unnecessary sun exposure and use a sunscreen
- avoid contact with the eyes, lips and mouth
- avoid contact with hair and dyed fabrics, which may be bleached by this product
Keep out of reach of children
- if swallowed, get medical help or contact a Poison Control Center right away.
Directions
- clean the skin thoroughly before applying this product
- work into a lather
- cover the entire affected area with a thin layer and rinse thoroughly one to three times daily
- pat dry
- because excessive drying of the skin may occur, start with one application daily, then graudally increase to two or three times daily if needed or as directed by a doctor
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day
- if going outside, apply sunscreen after using this product. If irritation or sensitivity develops, stop ise of both products and ask a doctor.
Inactive ingredients
Aqua, Cetearyl Alcohol, Decyl Glucoside, Dextrin, Glycerin, Sodium Chloride, Sodium Cocoyl Isethionate, Tetrasodium Glutamate Diacetate
| PANOXYL
benzoyl peroxide soap |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - The Original Bradford Soap Works Inc (001201045) |
Revised: 6/2023
Document Id: fd285e1d-01d9-2409-e053-6294a90a7a2e
Set id: fd285e1d-01d8-2409-e053-6294a90a7a2e
Version: 1
Effective Time: 20230602
Trademark Results [Panoxyl]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 PANOXYL 98758809 not registered Live/Pending |
Crown Laboratories, Inc. 2024-09-19 |
 PANOXYL 98730203 not registered Live/Pending |
Foshan Taodu E-commerce Co., Ltd 2024-09-03 |
 PANOXYL 72437359 0966901 Live/Registered |
STIEFEL LABORATORIES, INC. 1972-10-02 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.