CRENESSITY- crinecerfont capsule CRENESSITY- crinecerfont solution
Crenessity by
Drug Labeling and Warnings
Crenessity by is a Prescription medication manufactured, distributed, or labeled by Neurocrine Biosciences, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use CRENESSITY safely and effectively. See full prescribing information for CRENESSITY.
CRENESSITY™ (crinecerfont) capsules, for oral use
CRENESSITY™ (crinecerfont) oral solution
Initial U.S. Approval: 2024
INDICATIONS AND USAGE
CRENESSITY is a corticotropin-releasing factor type 1 receptor antagonist indicated as adjunctive treatment to glucocorticoid replacement to control androgens in adults and pediatric patients 4 years of age and older with classic congenital adrenal hyperplasia (CAH). (1)
DOSAGE AND ADMINISTRATION
- Continue glucocorticoid replacement therapy for adrenal insufficiency associated with CAH. (2.1)
-
Adults: 100 mg orally, twice daily with a meal in the morning and evening. (2.2)
-
Pediatric Patients (4 years of age and older): Weight-based dosage orally, twice daily with a meal in the morning and evening. (2.2)
- See Full Prescribing Information for complete dosage and administration information.
CONTRAINDICATIONS
Hypersensitivity to crinecerfont or any excipients of CRENESSITY. (4)
WARNINGS AND PRECAUTIONS
-
Hypersensitivity Reactions: Include throat tightness, angioedema, and generalized rash. If clinically significant hypersensitivity occurs, initiate appropriate therapy and discontinue CRENESSITY. (5.1)
- Risk of Acute Adrenal Insufficiency or Adrenal Crisis with Inadequate Concomitant Glucocorticoid Therapy: Continue glucocorticoids upon initiation of and during treatment with CRENESSITY. Do not reduce the glucocorticoid dose below the dose required for cortisol replacement. Any adjustment of daily glucocorticoid dosage after initiation of CRENESSITY should be performed under the supervision of a health care provider. Use glucocorticoid stress doses in cases of increased cortisol need (e.g., acute intercurrent illness, serious trauma, surgical procedures). (5.2)
ADVERSE REACTIONS
Adults: Most common adverse reactions (at least 4% for CRENESSITY and greater than placebo) are fatigue, headache, dizziness, arthralgia, back pain, decreased appetite, and myalgia. (6.1)
Pediatric Patients: Most common adverse reactions (at least 4% for CRENESSITY and greater than placebo) are headache, abdominal pain, fatigue, nasal congestion, and epistaxis. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Neurocrine Biosciences, Inc. at 877-641-3461 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 12/2024
- Continue glucocorticoid replacement therapy for adrenal insufficiency associated with CAH. (2.1)
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Information
2.2 Recommended Dosage and Administration
2.3 Dosage Modifications for Concomitant Use with Strong CYP3A4 Inducers
2.4 Dosage Modifications for Concomitant Use with Moderate CYP3A4 Inducers
2.5 Administration Instructions
2.6 Missed Doses
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Risk of Acute Adrenal Insufficiency or Adrenal Crisis with Inadequate Concomitant Glucocorticoid
Therapy6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Effects of Other Drugs on CRENESSITY
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Adults with Classic Congenital Adrenal Hyperplasia
14.2 Pediatric Patients with Classic Congenital Adrenal Hyperplasia
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Information
Patients receiving CRENESSITY should continue glucocorticoid replacement therapy for the adrenal insufficiency associated with congenital adrenal hyperplasia (see Warning and Precautions (5.2).
Androstenedione levels can be assessed beginning four weeks after CRENESSITY initiation to inform reduction in glucocorticoid dosage as clinically indicated. Do not reduce the glucocorticoid dosage below that required for replacement therapy.
2.2 Recommended Dosage and Administration
Recommended Dosage for Adults
The recommended CRENESSITY dosage for adults is 100 mg orally, twice daily with a meal in the morning and evening [see Clinical Pharmacology (12.3)].
Recommended Dosage for Pediatric Patients 4 Years of Age and Older
The recommended CRENESSITY dosage for pediatric patients 4 years of age and older is weight-based and administered orally, twice daily with a meal in the morning and evening (see Table 1) [see Clinical Pharmacology (12.3)].
Table 1: Recommended CRENESSITY Weight-Based Dosage for Pediatric Patients 4 Years of Age and Older
Weight Dosage Regimen with a Meal 10 kg to less than 20 kg 25 mg orally twice daily 20 kg to less than 55 kg 50 mg orally twice daily Greater than or equal to 55 kg 100 mg orally twice daily 2.3 Dosage Modifications for Concomitant Use with Strong CYP3A4 Inducers
Adults
In adults, increase the CRENESSITY dosage to 200 mg orally, twice daily with a meal in the morning and evening when used concomitantly with strong CYP3A4 inducers [see Drug Interactions (7.1)].
Pediatric Patients 4 Years of Age and Older
In pediatric patients, increase the CRENESSITY dosage with a meal in the morning and evening when used concomitantly with strong CYP3A4 inducers as shown in Table 2 [see Drug Interactions (7.1)].
Table 2: Dosage Increase of CRENESSITY for Use with Strong CYP3A4 Inducers in Pediatric Patients 4 Years of Age and Older
Weight Dosage Regimen with a Meal 10 kg to less than 20 kg 50 mg orally twice daily 20 kg to less than 55 kg 100 mg orally twice daily Greater than or equal to <55 kg 200 mg orally twice daily 2.4 Dosage Modifications for Concomitant Use with Moderate CYP3A4 Inducers
Adults
In adults, increase the CRENESSITY dosage to 200 mg orally with the evening meal when used concomitantly with moderate CYP3A4 inducers. The CRENESSITY dosage of 100 mg with the morning meal remains unchanged [see Drug Interactions (7.1)].
Pediatric Patients 4 Years of Age and Older
In pediatric patients, increase the CRENESSITY dosage with the evening meal when used concomitantly with moderate CYP3A4 inducers as shown in Table 3 [see Drug Interactions (7.1)].
Table 3. Dosage Increase of CRENESSITY for Use with Moderate CYP3A4 Inducers in Pediatric Patients 4 Years of Age and Older
Dosage Regimen with a Meal Weight Morning Dose Evening Dose 10 kg to less than 20 kg 25 mg orally 50 mg orally 20 kg to less than 55 kg 50 mg orally 100 mg orally Greater than or equal to 55 kg 100 mg orally 200 mg orally 2.5 Administration Instructions
Administer CRENESSITY orally, twice daily, with a meal in the morning and evening [see Clinical Pharmacology (12.3)].
CRENESSITY Capsules
Take CRENESSITY capsules orally and swallow whole with liquid.
CRENESSITY Oral Solution
Refer patients and/or caregivers to the Instructions for Use (IFU) for complete administration instructions.
Discard any unused CRENESSITY oral solution after 30 days of first opening the bottle [see How Supplied/Storage and Handling (16.2)].
-
3 DOSAGE FORMS AND STRENGTHS
CRENESSITY is available as:
- Capsules
- 25 mg oval, orange soft gelatin capsule printed with ‘WWV 25’ in black ink
- 50 mg oval, two-toned orange and gold soft gelatin capsule printed with ‘WWV 50’ in black ink
- 100 mg oblong, gold soft gelatin capsule printed with ‘WWV 100’ in black ink
- 25 mg oval, orange soft gelatin capsule printed with ‘WWV 25’ in black ink
- Oral Solution: 50 mg/mL in light yellow to orange, clear to slightly opalescent oral solution
- Capsules
-
4 CONTRAINDICATIONS
CRENESSITY is contraindicated in patients with hypersensitivity to crinecerfont or any excipients of CRENESSITY. Reactions have included throat tightness, angioedema, and generalized rash [see Warnings and Precautions (5.1)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
A hypersensitivity reaction, including throat tightness, angioedema, and generalized rash, occurred in a subject after 3 days of treatment with CRENESSITY.
If a clinically significant hypersensitivity reaction occurs, initiate appropriate therapy and discontinue CRENESSITY.
5.2 Risk of Acute Adrenal Insufficiency or Adrenal Crisis with Inadequate Concomitant Glucocorticoid
TherapyContinue glucocorticoids upon initiation of and during treatment with CRENESSITY. Do not reduce the glucocorticoid dose below the dose required for cortisol replacement.
Acute adrenal insufficiency or adrenal crisis, which can potentially be fatal or life-threatening, can occur in patients with underlying adrenal insufficiency who are on inadequate daily glucocorticoid doses, especially in situations associated with increased cortisol need, such as acute intercurrent illness, serious trauma, or surgical procedures.
Any adjustment of daily glucocorticoid dosage after initiation of CRENESSITY should be performed under the supervision of a health care provider. Use glucocorticoid stress doses in case of increased cortisol need (e.g., acute intercurrent illness, serious trauma, surgical procedures).
In the placebo-controlled clinical study of adults with classic CAH, the incidence of adrenal crisis was 1.6% in subjects treated with CRENESSITY and 0% in subjects treated with placebo. In the placebo-controlled clinical study of pediatric subjects with classic CAH, there were no events of adrenal crisis.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Hypersensitivity Reactions [see Warnings and Precautions (5.1)]
- Risk of Acute Adrenal Insufficiency or Adrenal Crisis with Inadequate Concomitant Glucocorticoid Therapy [see Warnings and Precautions (5.2)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adults with Congenital Adrenal Hyperplasia (CAH)
The safety of CRENESSITY in adults was assessed in Study 1, a randomized, double-blind, placebo-controlled study of 182 adults aged 18 to 58 years with classic CAH due to 21-hydroxylase deficiency. A total of 122 subjects received CRENESSITY 100 mg twice daily and 59 subjects received placebo twice daily for up to 24 weeks [see Clinical Studies (14.1)].
Adverse Reactions Leading to Discontinuation of Treatment
A total of 3% of CRENESSITY-treated subjects and no placebo-treated subjects discontinued treatment because of adverse reactions of restlessness, apathy, dyspepsia, nausea, and vomiting.
Commonly Observed Adverse Reactions
Adverse reactions that occurred in ≥4% of CRENESSITY-treated subjects and more frequently than in placebo-treated subjects are presented in Table 4.
Table 4: Adverse Reactions (≥4%) in Adults with Congenital Adrenal Hyperplasia Treated with CRENESSITY and Occurring More Frequently Than in Placebo-Treated Subjects
Adverse Reactions CRENESSITY
(N=122)
%Placebo
(N=59)
%Fatigue 25 15 Headache 16 15 Dizziness 8 3 Arthralgia 7 0 Back pain 6 3 Decreased appetite 4 2 Myalgia 4 3 Suicidal Ideation and Behavior
Study 1 excluded subjects with active suicidal ideation with intent or plan within the six months prior to screening and those with a history of suicidal behavior within the past year, based on the Columbia-Suicide Severity Rating Scale (C-SSRS) administered at screening. The C-SSRS was administered to subjects at regular intervals during the study. Three of 122 (2.5%) CRENESSITY-treated subjects reported suicidal ideation without method, intent or plan on the C-SSRS during the 24-week double-blind treatment period compared to 1 of 59 (1.7%) placebo-treated subjects. One of the three subjects receiving CRENESSITY and the placebo-treated subject reported a lifetime history of suicidal ideation.
One CRENESSITY-treated subject without a history of suicidal ideation or behavior attempted suicide during the open-label period after 320 days of treatment.
Laboratory Findings
Neutrophil count less than 2 x 103 cells/mcL occurred in 14% (17 of 120) of CRENESSITY-treated subjects, compared to 5% (3 of 58) of subjects in the placebo group. Neutrophil count less than 1 x 103 cells/mcL occurred in 0.8% (1 of 120) of CRENESSITY-treated subjects, compared to 1.7% subjects (1 of 58) in the placebo group.
Pediatric Patients with Congenital Adrenal Hyperplasia
The safety of CRENESSITY in pediatric patients was evaluated in Study 2, a randomized, double-blind placebo-controlled study of 103 pediatric subjects aged 4 to 17 years with classic CAH due to 21-hydroxylase deficiency. Pediatric subjects were randomized to receive CRENESSITY twice daily (N=69) or placebo (N=34) for 28 weeks, using weight-based dosing (50 mg twice daily via oral solution for subjects 20 to <55 kg, or 100 mg twice daily via oral capsules for subjects ≥55 kg) [see Clinical Studies (14.2)].
Adverse Reactions Leading to Discontinuation of Treatment
A total of 3% of CRENESSITY-treated subjects and no placebo-treated subjects discontinued treatment because of adverse reactions of abdominal pain, myalgia, and dizziness.
Commonly Observed Adverse Reactions
Adverse reactions that occurred at an incidence of ≥4% in CRENESSITY-treated pediatric subjects (50 mg twice daily or 100 mg twice daily) and greater than placebo are presented in Table 5.
Table 5: Adverse Reactions (≥ 4%) in Pediatric Subjects with Congenital Adrenal Hyperplasia Treated with CRENESSITY and Occurring More Frequently Than in Placebo-Treated Subjects
Adverse Reactions CRENESSITY
(N=69)
%Placebo
(N=33)
%Headache 25 6 Abdominal pain1 13 0 Fatigue 7 0 Nasal congestion 7 3 Epistaxis 4 0 1Abdominal pain includes: abdominal pain, abdominal pain upper and abdominal pain lower
Suicidal Ideation and Behavior
Study 2 excluded subjects with active suicidal ideation with intent or plan within six months prior to screening or those with a lifetime history of suicidal behavior based on the C-SSRS administered at screening. Four of 67 (6%) CRENESSITY-treated subjects and 0 of the 31 (0%) placebo-treated subjects reported suicidal ideation without method, intent or plan on the C-SSRS during the 28-week double-blind treatment period. Two of the four CRENESSITY-treated subjects reported a lifetime history of suicidal ideation. There were no completed suicides or suicide attempts.
Laboratory Findings
Neutrophil count less than 2 x 103 cells/mcL occurred in 37% (25 of 68) of CRENESSITY-treated subjects, compared to 16% (5 of 32) of subjects in the placebo group. Neutrophil count less than 1 x 103 cells/mcL occurred in 4% (3 of 68) of CRENESSITY-treated subjects, compared to no subjects (0 of 32) in the placebo group.
- Hypersensitivity Reactions [see Warnings and Precautions (5.1)]
-
7 DRUG INTERACTIONS
7.1 Effects of Other Drugs on CRENESSITY
Strong CYP3A4 Inducers
Increase CRENESSITY morning and evening dosages 2-fold when CRENESSITY is used concomitantly with a strong CYP3A4 inducer [see Dosage and Administration (2.3)].
Moderate CYP3A4 Inducers
Increase CRENESSITY evening dosage 2-fold when CRENESSITY is used concomitantly with a moderate CYP3A4 inducer. Do not increase the morning dosage [see Dosage and Administration (2.4)].
Mechanism of Drug Interaction and Clinical Effect
CRENESSITY is a CYP3A4 substrate. Concomitant use of CRENESSITY with a strong or moderate CYP3A4 inducer decreases crinecerfont exposure [see Clinical Pharmacology (12.3)], which may reduce CRENESSITY efficacy.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data from reports of pregnancy in clinical trials with CRENESSITY are insufficient to identify a drug-associated risk of major birth defects, miscarriage or other adverse maternal or fetal outcomes.
No developmental toxicity was observed in rats at 4-fold higher than human exposure at the maximum recommended human dose (MRHD) based on area under the concentration-time curve (AUC). Crinecerfont was associated with a low incidence of poly-malformations (craniofacial defects) in rabbits at 2-fold higher than human exposure at the MRHD. In a pre- and postnatal developmental toxicity study, no developmental toxicity was observed in rats at 4-fold higher than human exposure at the MRHD (see Data).
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
If CRENESSITY is administered during pregnancy, or if a patient becomes pregnant while receiving CRENESSITY, health care providers should report exposure to CRENESSITY by calling 1-855-CRNSITY (1-855-276-7489).
Data
Animal Data
Crinecerfont was administered orally to pregnant rabbits at doses of 100, 500, and 1000 mg/kg/day, and to pregnant rats at doses of 150, 500, and 2000 mg/kg/day during the period of organogenesis. No crinecerfont-related malformations were observed in rats at 4-fold higher than human exposure at the MRHD based on AUC. Low incidence of poly-malformations (craniofacial defects) and slightly lower mean fetal weights were observed in rabbits treated with crinecerfont at 2-fold higher than human exposure at the MRHD based on AUC.
In a pre- and postnatal developmental toxicity study, crinecerfont was administered orally to pregnant rats at doses of 15, 50, and 250 mg/kg/day during the period of organogenesis and lactation through Day 20 postpartum. No changes in pup mortality, growth, sexual maturation, behavior, mating and fertility, or ovarian and uterine parameters were observed. The exposure in dams at the No Observed Adverse Effect Level (NOAEL) of 250 mg/kg/day was approximately 4-fold higher than human exposure at the MRHD based on AUC.
8.2 Lactation
Risk Summary
There are no data on the presence of crinecerfont in human milk, the effects on the breastfed infant, or the effects on milk production. Crinecerfont is present in animal milk. (see Data). When a drug is present in animal milk, it is likely that the drug will be present in human milk. Infants exposed to CRENESSITY through breast milk should be monitored for signs of adrenal insufficiency such as weakness, decreased feeding and weight loss.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for CRENESSITY and any potential adverse effects on the breastfed child from CRENESSITY or from the underlying maternal condition.
Data
Crinecerfont was excreted in the milk of rats, with milk-to-plasma concentration ratios ranging from 1.5 to 12. No effects on postnatal development were observed in a pre- and postnatal development study, in which female rats were treated orally with up to 250 mg/kg/day (4-fold higher than human exposure at the MRHD based on AUC) during organogenesis through lactation.
8.4 Pediatric Use
The safety and effectiveness of CRENESSITY as adjunctive treatment to glucocorticoid replacement to control androgens have been established in pediatric patients 4 years of age and older with classic CAH. Use of CRENESSITY for this indication is supported by evidence from an adequate and well-controlled study of 103 pediatric subjects (Study 2), evidence from an adequate and well-controlled study in adults with CAH (Study 1), and pharmacokinetic data from adults and pediatric subjects [see Dosage and Administration (2.2), Warnings and Precautions (5.2), Adverse Reactions (6.1), Clinical Pharmacology (12.3), and Clinical Studies (14.2)].
The safety and effectiveness of CRENESSITY in pediatric patients less than 4 years of age have not been established.
8.5 Geriatric Use
The clinical trial of CRENESSITY in adults with classic CAH did not enroll subjects 65 years of age and older to determine whether they respond differently from younger subjects.
8.6 Renal Impairment
CRENESSITY is not recommended in patients with severe renal impairment or end-stage renal disease [see Clinical Pharmacology (12.3)].
-
11 DESCRIPTION
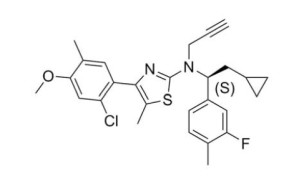
CRENESSITY contains crinecerfont, a selective corticotropin-releasing factor type 1 receptor antagonist, present as crinecerfont free base, with the chemical name, 2-thiazolamine, 4-(2-chloro-4-methoxy-5-methylphenyl)-N-[(1S)-2-cyclopropyl-1-(3-fluoro-4-methylphenyl)ethyl]-5-methyl-N-2-propyn-1-yl. Crinecerfont free base is the S-enantiomer with an enantiomeric excess of at least 99.7%. Its molecular formula is C27H28ClFN2OS, and its molecular weight is 483.04 g/mol with the following structure:
CRENESSITY Capsules
CRENESSITY capsules are intended for oral administration only. Each capsule contains 25 mg, 50 mg, or 100 mg of crinecerfont free base. Inactive ingredients include lauroyl polyoxyl-32 glycerides, medium chain triglycerides, propylene glycol dicaprylate/dicaprate, and Vitamin E polyethylene glycol succinate. The capsule shell contains gelatin, glycerin, red iron oxide, Sorbitol glycerin blend, titanium dioxide, and yellow iron oxide.
CRENESSITY Oral Solution
The oral solution formulation contains 50 mg/mL of crinecerfont free base. Inactive ingredients include butylated hydroxytoluene, medium-chain triglycerides, oleoyl polyoxyl glycerides, orange flavor, and saccharin.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Crinecerfont is a selective corticotropin-releasing factor (CRF) type 1 receptor antagonist. Crinecerfont blocks the binding of CRF to CRF type 1 receptors in the pituitary but not CRF type 2 receptors. Crinecerfont binding to CRF type 1 receptors inhibits adrenocorticotropic hormone (ACTH) secretion from the pituitary, thereby reducing ACTH-mediated adrenal androgen production.
12.2 Pharmacodynamics
Exposure-Response Relationships
Model based exposure-response analyses for adults and pediatric patients with CAH demonstrated that, within the studied exposures of CRENESSITY in Phase 3 trials, relatively flat exposure-response relationships were observed for androstenedione reduction from baseline to Week 4 for both adults and pediatric patients and percent glucocorticoid daily dose reduction from baseline to Week 24 for adults and to Week 28 for pediatric patients.
Adrenocorticotropic Hormone (ACTH) Reduction
In 8 adults with classic CAH (NCT0352886) who received the recommended CRENESSITY dosage for 2 weeks, the median percent reduction from baseline in ACTH was 62%.
In the Phase 3 clinical trials of adults and pediatric patients with classic CAH, administration of the recommended CRENESSITY dosage for 4 weeks during the initial glucocorticoid stable period led to a reduction in ACTH levels.
In Study 1, the median percent reduction from baseline to Week 4 in ACTH was 65%.
In Study 2, the median percent reduction from baseline to Week 4 in ACTH was 72%.
Cardiac Electrophysiology
At a dose 4 times the maximum approved recommended dosage, CRENESSITY does not prolong the QT interval to any clinically relevant extent.
12.3 Pharmacokinetics
Crinecerfont maximum plasma concentration (Cmax) and area under the time concentration curve (AUC0-24 hours) increases dose-proportionally over the approved recommended dosage range. Upon twice daily dosing, steady state conditions are achieved in approximately 7 days with an accumulation ratio of 1.4.
Absorption
Crinecerfont median time to reach Cmax (Tmax) is 4 hours following oral administration of CRENESSITY.
No clinically significant differences in crinecerfont Cmax or AUC were observed following administration of CRENESSITY oral capsules and oral solution.
Steady state exposures for crinecerfont in adults and pediatric patients following oral administration of CRENESSITY are presented in Table 6.
Table 6. Geometric Mean (CV%) for AUC24,ss and Cmax in Adults and Pediatric Patients with Classic Congenital Adrenal Hyperplasia Following Oral Administration of CRENESSITY
Parameter Geometric Mean (CV%) Study 1 (adults) Study 2 (pediatrics) AUC24hr,ss
(ng*h/mL)Dose: 100 mg bid 72846 (51%) Dose 100 mg bid (≥ 55 kg)
Dose 50 mg bid (≥ 20 to < 55 kg)74693 (48%)
47062 (51%)Cmax
(ng/mL)4231 (46%) Dose 100 mg bid (≥ 55 kg)
Dose 50 mg bid (≥ 20 to < 55 kg)4555 (43%)
2887 (48%)CV%, coefficient of variation expressed as a percentage; bid, twice daily; steady-state exposure parameters are generated from simulation of 500 subjects based on population pharmacokinetic modeling and weight band appropriate formulation
Effect of Food
Following administration of CRENESSITY capsule, crinecerfont Cmax increased 4.9-fold and AUC increased 3.3-fold with a high-fat meal (800 to 1000 calories, 50% fat), compared to fasted conditions [see Dosage and Administration (2.2)].
Following administration of CRENESSITY oral solution, crinecerfont Cmax increased 8.6-fold and AUC increased 8.3-fold with a high-fat meal (800 to 1000 calories, 50% fat), compared to fasted conditions [see Dosage and Administration (2.2)]
Distribution
Mean apparent volume of distribution (CV%) of crinecerfont in adults with CAH is 852 L (31%). Crinecerfont plasma protein binding is greater than or equal to 99.9%.
Elimination
Crinecerfont’s effective half-life is approximately 14 hours with a mean (CV%) apparent clearance of 3.5 L/h (37%).
Metabolism
Crinecerfont is metabolized primarily by CYP3A4 and to a lesser extent by CYP2B6 in vitro. Additionally, CYP2C8 and CYP2C19 may also have minor contributions to crinecerfont metabolism.
Excretion
Following a single oral 100 mg dose of radiolabeled crinecerfont, approximately 47.3% (2.7% as unchanged) of the dose was recovered in feces and 2% (amount as unchanged is undetectable) in urine.
Specific Populations
No clinically significant differences in the pharmacokinetics of crinecerfont were observed based on age (range: 5 to 54 years), sex (58.7% male), race (4.8% Asian, 9.7% Black, 77.5% White), mild to severe hepatic impairment (Child Pugh Class A to C), or mild to moderate renal impairment (estimated glomerular filtration rate: equal to or greater than 44 mL/min/1.73 m2; CKD-EPI 2009 formula for adults and bedside Schwartz formula for pediatric patients).
Crinecerfont has not been studied in patients with severe renal impairment or end-stage renal disease.
Drug Interaction Studies
Clinical Studies
Effect of Strong CYP3A4 Inducers on Crinecerfont: Crinecerfont Cmax decreased by 23% and AUC decreased by 62% following concomitant use with rifampin (strong CYP3A4 inducer) [see Dosage and Administration (2.3) and Drug Interactions (7.1)].
Effect of Strong CYP3A4 Inhibitors on Crinecerfont: Crinecerfont Cmax and AUC increased by 25% and 45%, respectively, when used concomitantly with ketoconazole (strong CYP3A4 inhibitor).
Effect of Crinecerfont on CYP3A Substrates: No clinically significant differences in the pharmacokinetics of midazolam (CYP3A4 substrate) were observed when co-administered with crinecerfont. Concomitant use of crinecerfont did not affect the pharmacokinetics of oral contraceptives containing ethinyl estradiol and levonorgestrel.
In Vitro Studies
Cytochrome P450 Enzymes: Crinecerfont does not inhibit CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, and CYP2E1. Crinecerfont does not induce CYP2C8, CYP3A4, CYP1A2 and CYP2B6.
Transporter Systems: Crinecerfont does not inhibit P-glycoprotein (P-gp), breast cancer resistant protein (BCRP), bile salt export pump (BSEP), organic anion transporting polypeptide (OATP) 1B1, OATP1B3, organic anion transporter (OAT)1, OAT3, organic cation transporter (OCT)2, multidrug and toxin extrusion protein (MATE)1, or MATE2-K.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
In a 2-year carcinogenicity study, rats were administered daily crinecerfont doses of 5, 15, and 100 mg/kg/day via oral gavage. An increased incidence of thyroid follicular cell combined adenomas and carcinomas was observed in female rats at the 100 mg/kg/day dose level (approximately 4-fold higher than human exposure at the MRHD based on AUC). Thyroid follicular cell tumors can occur in rats from excessive thyroid stimulating hormone (TSH) secondary to liver enzyme induction, which is not thought to commonly occur in humans. No evidence of increased tumorigenicity was seen in male rats up to approximately 5-fold higher than human exposure at the MRHD based on AUC or in female rats up to approximately 2-fold higher than human exposure at the MRHD based on AUC.
In a 6-month study in Tg.rasH2 mice, daily crinecerfont doses of 15, 50, 500, and 1500 mg/kg/day were administered via oral gavage. Crinecerfont did not increase the incidence of tumors in male or female transgenic mice (approximately 4-fold higher than human exposure at the MRHD based on AUC).
Mutagenesis
Crinecerfont was not mutagenic in the in vitro bacterial reverse mutation test (Ames) or clastogenic in the in vitro mammalian chromosomal aberrations assay in human peripheral blood lymphocytes or in the in vivo rat bone marrow micronucleus assay.
Impairment of Fertility
There were no crinecerfont-related effects on male or female fertility or female reproductive performance in rats treated orally with up to 1000 mg/kg/day crinecerfont at 2-fold higher than human exposure at the MRHD by AUC exposure.
-
14 CLINICAL STUDIES
14.1 Adults with Classic Congenital Adrenal Hyperplasia
The efficacy of CRENESSITY to reduce androgen levels and enable a reduced glucocorticoid dose while maintaining androgen control in adults with classic CAH was evaluated in a randomized, double-blind, placebo-controlled study (Study 1; NCT#04490915). This study enrolled 182 adults with classic CAH due to 21- hydroxylase deficiency on supraphysiological glucocorticoid doses and with androgen concentrations in the normal range or with inadequate androgen control.
Subjects were randomized to receive CRENESSITY 100 mg twice daily (N=122) or placebo (N=60) for 24 weeks. During the first 4 weeks of CRENESSITY treatment, subjects maintained a stable glucocorticoid regimen except for stress dosing as needed. During Weeks 4 to 12, the glucocorticoid dose was reduced as frequently as every 2 weeks without regard to androstenedione levels, with the goal to achieve a glucocorticoid dose of 8 to 10 mg/m2/day in hydrocortisone dose equivalents adjusted for body surface area by Week 12. From Weeks 12 to 20, the glucocorticoid dose was further adjusted, if needed, to achieve androstenedione control by Week 24.
The mean (range) age was 31 (18 to 58) years, 51% were male, 90% were White, and 8% were Hispanic or Latino. At baseline, the mean (SD) glucocorticoid total daily dose in hydrocortisone equivalents was 32 (9) mg/day (18 [5] mg/m2/day), with mean (SD) androstenedione levels of 620 (729) ng/dL prior to the morning glucocorticoid dose.
The efficacy of CRENESSITY was assessed by the least-squares (LS) mean (SEM) percent change from baseline in the total glucocorticoid daily dose while androstenedione was controlled (≤120% of baseline or ≤upper limit of normal [ULN]) after 24 weeks. The LS mean percent change from baseline in daily glucocorticoid dose was statistically significantly greater in the CRENESSITY group at -27% compared to -10% in the placebo group, as shown in Table 7.
Table 7: Primary Change From Baseline in Glucocorticoid Daily Dose While Maintaining Androstenedione Control at Week 24 in Adults with Classic Congenital Adrenal Hyperplasia (Study 1)
Treatment Group Mean (SD) Baseline (mg/m2/day) LS Mean (SEM) Percent Change From Baseline (%) Placebo-Subtracted LS Mean Difference (95%
CI)(%)Glucocorticoid Daily Dose* CRENESSITY
N=12218 (5) -27 (2) -17
(-24, -10)
p<0.0001Placebo
N=6018 (6) -10 (3) CI=confidence interval; LS mean=least-squares mean; SD=standard deviation; SEM=standard error of the mean
* In hydrocortisone equivalents (4x equivalency factor for (methyl)predniso(lo)ne, 60x for dexamethasone) adjusted for body surface area.At Week 24, there was a statistically significantly greater percentage of subjects achieving a reduction to a physiologic glucocorticoid daily dose (≤11 mg/m2/day hydrocortisone equivalents) while androstenedione was controlled (≤120% of baseline or ≤ULN) with CRENESSITY compared to placebo (63% vs 18%, p<0.0001).
At Week 4, following a treatment period at a stable glucocorticoid dose regimen, the LS mean change from baseline in serum androstenedione in the CRENESSITY group was statistically significantly different at -299 ng/dL compared to the LS mean increase from baseline in the placebo group of 46 ng/dL, as shown in Table 8.
Table 8: Change From Baseline in Serum Androstenedione (ng/dL) at Week 4* in Adults with Classic Congenital Adrenal Hyperplasia(Study 1)
Treatment Group Mean (SD) Baseline LS Mean (SEM) Change from Baseline Placebo-subtracted LS Mean Difference (95% CI) Serum Androstenedione (ng/dL)† CRENESSITY
N=122634
(796)-299
(38)-345
(-457, -232)
p<0.0001Placebo
N=60590
(572)46
(51)CI=confidence interval; LS mean=least-squares mean; SD=standard deviation; SEM=standard error of the mean
* End of glucocorticoid stable period.
† Obtained prior to the morning glucocorticoid dose.
14.2 Pediatric Patients with Classic Congenital Adrenal Hyperplasia
The efficacy of CRENESSITY to improve androgen control and enable a reduced glucocorticoid dose while maintaining androgen control in pediatric patients with classic CAH was evaluated in a Phase 3 randomized, double-blind, placebo-controlled study (Study 2; NCT#04806451). This study enrolled 103 pediatric subjects 4 to 17 years of age with classic CAH due to 21-hydroxylase deficiency and inadequate androgen control on supraphysiological glucocorticoid doses.
Subjects were randomized to receive either CRENESSITY twice daily (N=69) or placebo (N=34) for 28 weeks, using weight-based dosing (50 mg twice daily via oral solution for subjects 20 to <55 kg [CRENESSITY N=37; placebo N=14 ], or 100 mg twice daily via oral capsules for subjects ≥55 kg [CRENESSITY N=32; placebo N=20]).
During the first 4 weeks of CRENESSITY treatment, subjects were maintained on a stable glucocorticoid regimen except for stress dosing, as needed. The primary efficacy endpoint was the change from baseline in serum androstenedione at Week 4. From Weeks 4 to 20, the glucocorticoid dose could be reduced as frequently as every 4 weeks provided androstenedione levels were controlled. The goal was to achieve a glucocorticoid dose of 8 to 10 mg/m2/day (hydrocortisone dose equivalents adjusted for body surface area) by Week 28 while maintaining androstenedione control.
The mean (range) age was 12 (4 to 17) years, 41% were Tanner Stage 1 or 2, 52% were male, 63% were White, and 11% were Hispanic or Latino. With respect to concurrent glucocorticoid use at baseline, 92% of patients were receiving hydrocortisone alone and 8% were receiving prednisone [or equivalent] (with or without hydrocortisone). At baseline, subjects were receiving a mean (SD) glucocorticoid total daily dose in hydrocortisone equivalents of 16 (4) mg/m2/day, and had a mean (SD) androstenedione level of 431 (461) ng/dL and mean (SD) serum 17-hydroxyprogesterone level of 8682 (6847) ng/dL prior to the morning glucocorticoid dose.
At Week 4, following a treatment period at a stable glucocorticoid dose regimen, the LS mean reduction from baseline in serum androstenedione in the CRENESSITY group was statistically significantly different at -197 ng/dL compared to the increase of 71 ng/dL in the placebo group, as shown in Table 9.
Table 9: Change From Baseline in Serum Androstenedione (ng/dL) at Week 4* in Pediatric Subjects with Classic Congenital Adrenal Hyperplasia (Study 2)
Treatment Group Mean (SD) Baseline LS Mean (SEM) Change from Baseline Placebo-Subtracted LS Mean Difference (95% CI) Serum Androstenedione (ng/dL) † CRENESSITY
N=69405
(464)-197
(40)-268
(-403, -132)
p=0.0002Placebo
N=34483
(456)71
(56)CI=confidence interval; LS mean=least-squares mean; SD=standard deviation; SEM=standard error of the mean
* End of glucocorticoid stable period.
†Obtained prior to the morning glucocorticoid dose.
At Week 4, following a treatment period at a stable glucocorticoid regimen, the LS mean reduction (SEM) from baseline in serum 17-hydroxyprogesterone in the CRENESSITY group was statistically significantly different at -5865 (572) ng/dL compared to the increase of 556 (818) ng/dL in the placebo group (LS Mean Treatment Difference -6421, 95% CI -8387, -4454, p<0.0001).
The LS mean percent change from baseline in the total glucocorticoid daily dose while androstenedione was controlled (≤120% of baseline or ≤ULN) at Week 28 in the CRENESSITY group was statistically significantly different at -18% compared to the increase of 6% in the placebo group, as shown in Table 10.
Table 10: Percent Change From Baseline in Glucocorticoid Daily Dose While Maintaining
Androstenedione Control at Week 28 in Pediatric Subjects with Classic Congenital Adrenal Hyperplasia (Study 2)Treatment Group Mean (SD) Baseline
(mg/m2/day)LS Mean (SEM) Percent Change from Baseline
(%)Placebo-subtracted LS Mean Difference
(95% CI)
(%)Glucocorticoid Daily Dose* CRENESSITY
N=6917
(4)-18
(2)-24
(-30, -17)
p<0.0001Placebo
N=3416
(3)6
(3)CI=confidence interval; LS mean=least-squares mean; SD=standard deviation; SEM=standard error of the mean;
*In hydrocortisone equivalents (4x equivalency factor for (methyl)predniso(lo)ne) adjusted for body surface area. -
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
CRENESSITY Capsules
CRENESSITY (crinecerfont) capsules are available in the doses shown in Table 11.
Table 11. CRENESSITY Capsule Information
Capsule Strength Capsule Color/Shape Capsule Marking Quantity in bottle NDC Number 25 mg Oval, orange soft gelatin capsules Printed with ‘WWV 25’ in black ink 60 70370-5025-1 50 mg Oval, two-toned orange and gold soft gelatin capsules Printed with ‘WWV 50’ in black ink 60 70370-5050-1 100 mg Oblong, gold soft gelatin capsules Printed with ‘WWV 100’ in black ink 30 70370-5100-1 CRENESSITY Oral Solution 50 mg/mL is a light yellow to orange, orange-flavored liquid. The amber polyethylene terephthalate (PET) bottle contains 30 mL oral solution (NBC 70370-5250-1).
16.2 Storage and Handling
CRENESSITY Capsules
Store at 15°C to 25°C (59°F to 77°F).
Packaged in child-resistant HDPE bottles. Do not freeze.
CRENESSITY Oral Solution
Store and dispense in original container. Store CRENESSITY Oral Solution in an upright position.
Store unopened bottles under refrigeration at 2°C to 8°C (36°F to 46°F). Do not freeze.
Once a bottle is opened for use, it may be stored under refrigeration at 2°C to 8°C (36°F to 46°F) or at room temperature (15°C to 25°C [59°F to 77°F]) for up to 30 days. Discard any unused oral solution after 30 days of first opening the bottle.
Packaged in child-resistant PET bottles.
-
17 PATIENT COUNSELING INFORMATION
Advise the patients and/or caregivers to read the FDA-approved patient labeling (Patient Information leaflet and CRENESSITY oral solution IFU, if applicable).
Administration Information
Counsel patients that CRENESSITY must be taken with a meal, without regard to fat or caloric content [see Dosage and Administration (2.2) and Clinical Pharmacology (12.3)].
Drug Interactions
Inform patients that the CRENESSITY dosage will need to be increased if they are taking strong or moderate CYP3A4 inducers [see Dosage and Administration (2.3, 2.4), Drug Interactions (7.1)].
Hypersensitivity Reactions
Advise patients to seek prompt medical attention if signs or symptoms of hypersensitivity reactions occur. Advise patients who have had signs or symptoms of systemic hypersensitivity reactions to CRENESSITY that they should not receive CRENESSITY [see Contraindications (4) and Warnings and Precautions (5.1)].
Acute Adrenal Insufficiency or Adrenal Crisis with Inadequate Concomitant Glucocorticoid Therapy
Inform patients that they should continue glucocorticoids when taking CRENESSITY. Counsel patients that any adjustment of glucocorticoid doses should be done under the guidance of their health care provider. Counsel patients about the continued need for stress dose glucocorticoids during times of increased cortisol need (e.g., during illness) [see Warnings and Precautions (5.2)].
Pregnancy
Advise women who are exposed to CRENESSITY during pregnancy that there is a pregnancy safety study [see Use in Specific Populations (8.1)].
For information on CRENESSITY, visit www.CRENESSITY.com or call 1-855-CRNSITY
(1-855-276-7489).Distributed by: Neurocrine Biosciences, Inc., San Diego, CA 92130, U.S.A.
CRENESSITY is a trademark of Neurocrine Biosciences, Inc.
-
PATIENT PACKAGE INSERT
PATIENT INFORMATION
CRENESSITY® (kreh NEH sih tee)
(CRINECERFONT)
CAPSULES AND ORAL SOLUTIONWhat is CRENESSITY?
CRENESSITY is a prescription medicine used together with glucocorticoids (steroids) to control androgen (testosterone-like hormone) levels in adults and children 4 years of age and older with classic congenital adrenal hyperplasia (CAH).
It is not known if CRENESSITY is safe and effective in children younger than 4 years of age.Do not take CRENESSITY if you: - are allergic to crinecerfont, or any of the ingredients in CRENESSITY. See the end of this Patient Information leaflet for a complete list of ingredients in CRENESSITY.
Before taking CRENESSITY, tell your health care provider about all of your medical conditions, including if you:
- are pregnant or plan to become pregnant. It is not known if CRENESSITY will harm your unborn baby. If you become pregnant while taking CRENESSITY, tell your health care provider right away. There is a pregnancy safety study for women who become pregnant during treatment with CRENESSITY.
- are breastfeeding or plan to breastfeed. It is not known if CRENESSITY passes into your breast milk. Talk to your health care provider about the best way to feed your baby during treatment with CRENESSITY.
Taking CRENESSITY with certain other medicines may cause your CRENESSITY to not work as well. Do not start any new medicines while taking CRENESSITY without talking to your health care provider first.How should I take CRENESSITY? - Take CRENESSITY exactly as your health care provider tells you to. Your health care provider will tell you how much CRENESSITY to take and when to take it.
- Do not stop taking CRENESSITY without talking to your health care provider first.
- While your health care provider may lower your glucocorticoids (steroids), you should continue to take glucocorticoids (steroids) to treat adrenal insufficiency, including stress dose steroids (such as when your body may be under stress from fever, infection or surgery). Do not change your daily glucocorticoid (steroid) dose without talking to your health care provider. See “What are the possible side effects of CRENESSITY?”
- Take CRENESSITY by mouth 2 times each day, in the morning and evening with a meal.
- Swallow CRENESSITY capsules whole with liquid. Tell your health care provider if you cannot swallow the capsule whole. Your healthcare provider may need to switch you to CRENESSITY oral solution.
- If your health care provider prescribes CRENESSITY oral solution, you should use the oral dosing syringe that your pharmacist will give you to measure and take the correct dose. See the Instructions for Use for CRENESSITY oral solution for more information.
- If you miss a dose of CRENESSITY, take it as soon as you remember (even if it is almost time for your next dose). Take the next dose at your regular time.
What are the possible side effects of CRENESSITY?
CRENESSITY may cause serious side effects, including:
-
Allergic reactions. Symptoms of an allergic reaction include tightness of the throat, trouble breathing or swallowing, swelling of the lips, tongue, or face, and rash. If you have an allergic reaction to CRENESSITY, get emergency medical help right away and stop taking CRENESSITY.
- Risk of Sudden Adrenal Insufficiency or Adrenal Crisis with Too Little Glucocorticoid (Steroid) Medicine. Sudden adrenal insufficiency or adrenal crisis can happen in people with congenital adrenal hyperplasia who are not taking enough glucocorticoid (steroid) medicine. You should continue taking your glucocorticoid (steroid) medicine during treatment with CRENESSITY. Certain conditions such as infection, severe injury, or shock may increase your risk for sudden adrenal insufficiency or adrenal crisis. Tell your health care provider if you get a severe injury, infection, illness, or have any planned surgery during treatment with CRENESSITY. Your health care provider may need to change your dose of glucocorticoid (steroid) medicine.
tiredness back pain headache decreased appetite dizziness muscle pain joint pain The most common side effects of CRENESSITY in children include: headache nasal congestion stomach pain nose bleeds tiredness These are not all the possible side effects of CRENESSITY. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. How should I store CRENESSITY?
- Store CRENESSITY capsules at room temperature between 59°F to 77°F (15°C to 25°C ). Do not freeze.
- Store unopened CRENESSITY oral solution refrigerated between 36° to 46°F (2° to 8°C ). Do not freeze.
- Store in the original container in an upright position.
- After opening the bottle, CRENESSITY oral solution may be stored in the refrigerator or at room temperature between 59°F to 77°F (15°C to 25°C ) for up to 30 days.
- Use CRENESSITY oral solution within 30 days of first opening the bottle. Throw away (disopose of) any unused medicine after 30 days.
- Store in the original container in an upright position.
General information about the safe and effective use of CRENESSITY.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use CRENESSITY for a condition for which it was not prescribed. Do not give CRENESSITY to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or health care provider for information about CRENESSITY that is written for health professionals.What are the ingredients in CRENESSITY?
Active ingredient: crinecerfont
Inactive ingredients:
- CRENESSITY capsules: lauroyl polyoxyl-32 glycerides, medium chain triglycerides, propylene glycol dicaprylate/dicaprate, and Vitamin E polyethylene glycol succinate. The capsule shell contains gelatin, glycerin, red iron oxide, Sorbitol glycerin blend, titanium dioxide, yellow iron oxide.
- CRENESSITY oral solution: butylated hydroxytoluene, medium-chain triglycerides, oleoyl polyoxyl glycerides, orange flavor, and saccharin.
For more information, go to www.CRENESSITY.com or call 1-855-CRNSITY (1 855-276-7489).This Patient Information has been approved by the U.S. Food and Drug Administration Issued: 12/2024
-
INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE
CRENESSITYTM (kreh NEH sih tee)(crinecerfont)
oral solution
50 mg/mL
Read this Instructions for Use for important information you need to know before taking CRENESSITY oral solution for the first time and each time you get a refill. There may be new information. This information does not take the place of talking to your health care provider about your medical condition or treatment.
Important information:
- Take CRENESSITY oral solution exactly as your health care provider tells you to. Your health care provider will tell you how much CRENESSITY oral solution to take and when to take it.
- CRENESSITY oral solution should be taken with a meal in the morning and evening.
- Always use the oral syringe provided by your pharmacist to make sure you measure the right amount of CRENESSITY oral solution.
- Use CRENESSITY oral solution within 30 days of first opening the bottle. After 30 days of first opening the bottle, throw away (dispose of) any CRENESSITY oral solution that has not been used.
- Do not stop taking CRENESSITY oral solution without talking to your health care provider first.
- See the Patient Information Leaflet that comes with CRENESSITY for more information.
Supplies not included in the package:
- Press-in bottle adapter
- Oral syringes
You must get a press-in bottle adapter and oral syringe from your pharmacist. Your pharmacist can help you choose the right press-in bottle adapter and oral syringes to use with CRENESSITY oral solution.
Call your pharmacist right away if you do not have a press-in bottle adapter and the right oral syringe to use with your CRENESSITY oral solution.
How should I prepare and take CRENESSITY oral solution?
1. Gather the supplies (see Figure A):
CRENESSITY oral solution bottle
Press-in bottle adapter
Oral syringe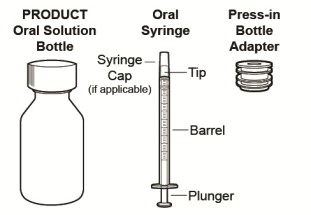
Figure A
2. Inspect CRENESSITY.
- Check the pharmacy label to make sure the medicine name and dose are correct.
- Check the oral solution bottle, oral syringe, and press-in bottle adapter for signs of damage.
3. Prepare CRENESSITY oral solution.
a. Remove the child-resistant cap by pressing it down while twisting it to the left (counterclockwise) (See Figure B).
b. Carefully puncture and peel off the seal from the bottle (See Figure C).
c. First time use of the bottle only. While holding the bottle firmly with one hand, use the thumb on your other hand to push the press-in bottle adapter into the bottle as far as it will go using constant pressure (see Figure D).
Do not remove the press-in bottle adapter after it has been inserted.
Figure B
Figure C
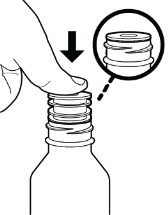
Figure D
4. Prepare the dose.
a. Push in the plunger of the oral syringe as far as it will go (see Figure E).
If the oral syringe comes with a cap, pull off the syringe cap.
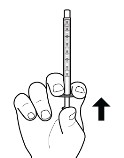
Figure Eb. Insert the tip of the oral syringe into the press-in bottle adapter, then with the oral syringe still inserted, turn the bottle upside down (see Figure F).
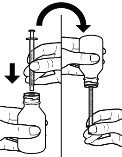
Figure Fc. Slowly pull the plunger of the oral syringe until the part of the plunger that is touching the liquid is even with the marking of your prescribed dose (see Figure G).
Note: Slowly push the plunger to return any large air bubbles back to the bottle and repeat the prior step until the air bubbles are gone.
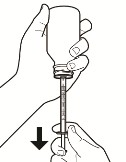
Figure G
d. When you have measured the correct amount of CRENESSITY, keep the oral syringe inserted in the bottle and turn the bottle and oral syringe right side up. Hold the oral syringe from the middle, then carefully remove it from the bottle (see Figure H).
Do not touch the plunger to avoid oral solution accidentally coming out of the syringe before you are ready to give the medicine.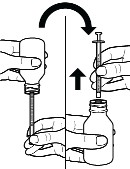
Figure H
5. Give a dose of CRENESSITY oral solution.
a. Place the tip of the oral syringe inside your mouth and point it towards the inside of the cheek.
b. Slowly push the plunger all the way down to give the full dose (see Figure I).
c. Put the child-resistant cap back on the bottle with the press-in bottle adapter still inserted by turning the cap to the right (clockwise).
Do not remove the press-in bottle adapter. The child-resistant cap will fit over it.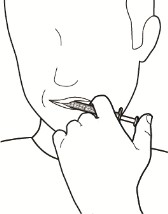
Figure I
6.Rinse and store the oral syringe.
a. Remove the plunger from the oral syringe.
b. Rinse both parts with water (see Figure J).
c. Push plunger back into the oral syringe to remove any large droplets of water.
d. Remove the plunger from the oral syringe and allow these separated parts to air dry thoroughly on a clean surface.
e. Push the dried plunger back into the dried oral syringe.
f. Store the oral syringe in a clean, dry place.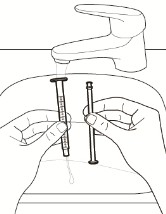
Figure J
How should I store CRENESSITY oral solution? - Store CRENESSITY oral solution in the original container in an upright position.
- Store the unopened CRENESSITY oral solution bottle in the refrigerator at 36°F to 46°F (2°C to 8°C (2°C to 8°C ). Do not freeze.
- After opening the bottle, CRENESSITY oral solution can be stored in the refrigerator or at room temperature between 59°F to 77°F (15°C to 25°C) for up to 30 days.
- Use CRENESSITY oral solution within 30 days of first opening the bottle. Throw away (dispose of) any unused medicine after 30 days.
- Keep CRENESSITY oral solution and all medicines out of the reach of children.
Distributed by: Neurocrine Biosciences, Inc., San Diego, CA 92130, U.S.A
For information, visit www.CRENESSITY.com or call 1-855-CRNSITY (1 855-276-7489).This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Issued: 12/2024
- Take CRENESSITY oral solution exactly as your health care provider tells you to. Your health care provider will tell you how much CRENESSITY oral solution to take and when to take it.
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CRENESSITY
crinecerfont capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 70370-5025 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CRINECERFONT (UNII: MFT24BX55I) (CRINECERFONT - UNII:MFT24BX55I) CRINECERFONT 25 mg Inactive Ingredients Ingredient Name Strength LAUROYL PEG-32 GLYCERIDES (UNII: H5ZC52369M) PROPYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: O4446S9CRA) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) VITAMIN E POLYETHYLENE GLYCOL SUCCINATE (UNII: O03S90U1F2) GELATIN (UNII: 2G86QN327L) SORBITOL (UNII: 506T60A25R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) Product Characteristics Color orange Score no score Shape OVAL Size 13mm Flavor Imprint Code WWV;25 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70370-5025-1 60 in 1 BOTTLE; Type 0: Not a Combination Product 12/13/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA218808 12/13/2024 CRENESSITY
crinecerfont capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 70370-5050 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CRINECERFONT (UNII: MFT24BX55I) (CRINECERFONT - UNII:MFT24BX55I) CRINECERFONT 50 mg Inactive Ingredients Ingredient Name Strength LAUROYL PEG-32 GLYCERIDES (UNII: H5ZC52369M) PROPYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: O4446S9CRA) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) VITAMIN E POLYETHYLENE GLYCOL SUCCINATE (UNII: O03S90U1F2) GELATIN (UNII: 2G86QN327L) SORBITOL (UNII: 506T60A25R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) Product Characteristics Color orange (two-toned orange and gold) Score no score Shape OVAL Size 16mm Flavor Imprint Code WWV;50 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70370-5050-1 60 in 1 BOTTLE; Type 0: Not a Combination Product 12/13/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA218808 12/13/2024 CRENESSITY
crinecerfont capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 70370-5100 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CRINECERFONT (UNII: MFT24BX55I) (CRINECERFONT - UNII:MFT24BX55I) CRINECERFONT 100 mg Inactive Ingredients Ingredient Name Strength LAUROYL PEG-32 GLYCERIDES (UNII: H5ZC52369M) PROPYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: O4446S9CRA) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) VITAMIN E POLYETHYLENE GLYCOL SUCCINATE (UNII: O03S90U1F2) GELATIN (UNII: 2G86QN327L) SORBITOL (UNII: 506T60A25R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) Product Characteristics Color yellow (gold) Score no score Shape CAPSULE Size 22mm Flavor Imprint Code WWV;100 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70370-5100-1 30 in 1 BOTTLE; Type 0: Not a Combination Product 12/13/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA218808 12/13/2024 CRENESSITY
crinecerfont solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 70370-5250 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CRINECERFONT (UNII: MFT24BX55I) (CRINECERFONT - UNII:MFT24BX55I) CRINECERFONT 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PEG-5 OLEATE (UNII: 0240V77G50) SACCHARIN (UNII: FST467XS7D) Product Characteristics Color yellow (light yellow to orange) Score Shape Size Flavor ORANGE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70370-5250-1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 12/13/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA218820 12/13/2024 Labeler - Neurocrine Biosciences, Inc. (800981276)
Trademark Results [Crenessity]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CRENESSITY 98404854 not registered Live/Pending |
Neurocrine Biosciences, Inc. 2024-02-14 |
 CRENESSITY 97028747 not registered Live/Pending |
Neurocrine Biosciences, Inc. 2021-09-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.



