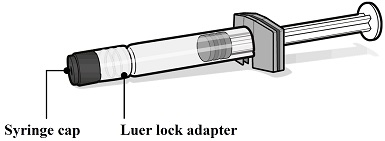
PENBRAYA- meningococcal groups a, b, c, w, and y vaccine kit
PENBRAYA by
Drug Labeling and Warnings
PENBRAYA by is a Other medication manufactured, distributed, or labeled by Pfizer Laboratories Div Pfizer Inc, Pfizer Health AB, Pfizer Inc, Wyeth Pharmaceutical Division of Wyeth Holdings LLC, Pfizer Ireland Pharmaceuticals, Pfizer Manufacturing Austria GmbH, Pfizer Manufacturing Belgium NV. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use PENBRAYA safely and effectively. See full prescribing information for PENBRAYA.
PENBRAYA® (Meningococcal Groups A, B, C, W, and Y Vaccine), suspension for intramuscular injection
Initial U.S. Approval: 2023INDICATIONS AND USAGE
PENBRAYA is indicated for active immunization to prevent invasive disease caused by Neisseria meningitidis serogroups A, B, C, W, and Y. PENBRAYA is approved for use in individuals 10 through 25 years of age. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
PENBRAYA is a suspension for injection. A single dose after reconstitution is approximately 0.5 mL. (3)
CONTRAINDICATIONS
Severe allergic reaction (e.g., anaphylaxis) to any component of PENBRAYA. (4)
ADVERSE REACTIONS
The most commonly reported (≥15%) solicited adverse reactions after Dose 1 and Dose 2, respectively, were pain at the injection site (89% and 84%), fatigue (52% and 48%), headache (47% and 40%), muscle pain (26% and 23%), injection site redness (26% and 23%), injection site swelling (25% and 24%), joint pain (20% and 18%), and chills (20% and 16%). (6)
To report SUSPECTED ADVERSE REACTIONS, contact Pfizer Inc. at 1-800-438-1985 or VAERS at 1-800-822-7967 or http://vaers.hhs.gov.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 11/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dose and Schedule
2.2 Preparation
2.3 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Management of Acute Allergic Reactions
5.2 Syncope
5.3 Altered Immunocompetence
5.4 Limitations of Vaccine Effectiveness
5.5 Tetanus Immunization
5.6 Guillain-Barré Syndrome
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Immunogenicity
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage Before Reconstitution
16.3 Storage After Reconstitution
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
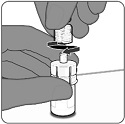
For intramuscular use only.
2.2 Preparation
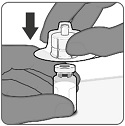
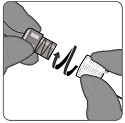
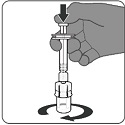
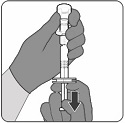
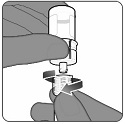
PENBRAYA is supplied in a kit that includes a vial of Lyophilized MenACWY Component (a sterile white powder), a prefilled syringe containing the MenB Component and a vial adapter.
To form PENBRAYA, reconstitute the Lyophilized MenACWY Component with the MenB Component as described in the instructions below.
2.3 Administration
For intramuscular use only.
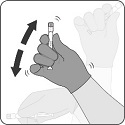
After reconstitution, PENBRAYA is a homogeneous white suspension. If the vaccine is not a homogenous suspension, shake to resuspend prior to administration. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Discard if either condition is present.
Administer PENBRAYA immediately or store between 2°C and 30°C (36°F and 86°F) and use within 4 hours. Discard reconstituted vaccine if not used within 4 hours.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Do not administer PENBRAYA to individuals with a history of severe allergic reaction (e.g., anaphylaxis) to any component of PENBRAYA [see Description (11)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Management of Acute Allergic Reactions
Appropriate medical treatment used to manage immediate allergic reactions must be immediately available in the event an anaphylactic reaction occurs following administration of PENBRAYA.
5.2 Syncope
Syncope (fainting) may occur in association with administration of injectable vaccines, including PENBRAYA. Procedures should be in place to avoid injury from fainting.
5.3 Altered Immunocompetence
Reduced Immune Response
Some individuals with altered immunocompetence may have reduced immune responses to PENBRAYA.
Complement Deficiency
Individuals with certain complement deficiencies and individuals receiving treatment that inhibits terminal complement activation are at increased risk for invasive disease caused by N. meningitidis groups A, B, C, W, and Y, even if they develop antibodies following vaccination with PENBRAYA [see Clinical Pharmacology (12.1)].
5.4 Limitations of Vaccine Effectiveness
Vaccination with PENBRAYA may not protect all vaccine recipients.
5.5 Tetanus Immunization
Vaccination with PENBRAYA does not substitute for vaccination with a tetanus toxoid containing vaccine to prevent tetanus.
5.6 Guillain-Barré Syndrome
Guillain-Barré syndrome (GBS) has been reported in temporal relationship following administration of another U.S.-licensed meningococcal quadrivalent polysaccharide conjugate vaccine. The decision by the healthcare professional to administer PENBRAYA to persons with a history of GBS should take into account the expected benefits and potential risks.
-
6 ADVERSE REACTIONS
The most commonly reported (≥15%) solicited adverse reactions after Dose 1 and Dose 2, respectively, were pain at the injection site (89% and 84%), fatigue (52% and 48%), headache (47% and 40%), muscle pain (26% and 23%), injection site redness (26% and 23%), injection site swelling (25% and 24%), joint pain (20% and 18%), and chills (20% and 16%).
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a vaccine cannot be directly compared to rates in the clinical trials of another vaccine and may not reflect the rates observed in practice.
The safety of PENBRAYA in individuals 10 through 25 years of age was evaluated in 3 clinical studies (2 active-controlled and 1 non-controlled study). In the controlled studies, 2306 participants received at least 1 dose of PENBRAYA. Of 946 participants who had previously received a meningococcal conjugate vaccine (categorized as MenACWY conjugate vaccine-exposed), 802 participants had received 1 dose of any U.S.-licensed Meningococcal Groups A, C, W, and Y conjugate vaccine, 51 participants had received a non-U.S.-licensed monovalent Meningococcal Group C conjugate vaccine (MenC conjugate vaccine), and 93 participants had received an unspecified U.S.-licensed or non-U.S.-licensed MenACWY conjugate or a MenC conjugate vaccine at least 4 years prior to enrollment. In the non-controlled study, 300 participants received a single dose of PENBRAYA.
Study 1 (NCT04440163) was an active-controlled, observer-blinded, multicenter study in which participants 10 through 25 years of age in the U.S. and Europe received at least 1 dose of PENBRAYA (N=1763) or Meningococcal Group B Vaccine (Trumenba) (N=650) at 0 and 6 months. Meningococcal Groups A, C, Y, and W-135 Oligosaccharide Diphtheria CRM197 Conjugate Vaccine (MenACWY-CRM, GSK Vaccines, SRL) was concomitantly administered with Trumenba at Month 0. All participants were MenB vaccine-naïve. Both MenACWY conjugate vaccine-naïve and MenACWY conjugate vaccine-exposed participants were part of the study.
Study 2 (NCT03135834) was an active-controlled, observer-blinded, multicenter study in which participants 10 through 25 years of age in the U.S. and Europe received at least 1 dose of PENBRAYA (N=543) or Trumenba (N=1057) at 0 and 6 months. MenACWY-CRM was co-administered with Trumenba at Month 0. All participants were MenB vaccine-naïve. Both MenACWY conjugate vaccine-naïve and MenACWY conjugate vaccine-exposed participants were part of this study.
Study 3 (NCT04440176) was a descriptive non-controlled study in which participants 11 through 14 years of age in the U.S. received PENBRAYA 12 months apart. All participants were naïve to any meningococcal vaccine.
In Study 1 and Study 2, solicited local and systemic adverse reactions were monitored for 7 days after study vaccination using an electronic diary. In all studies, spontaneous reports of adverse events (AEs) were collected through at least 1 month after the last vaccination, and through 6 months after the last vaccination for serious adverse events (SAEs).
In controlled studies, demographic characteristics were generally similar with regard to gender, race, and ethnicity among participants who received PENBRAYA and those who received control (Trumenba and MenACWY-CRM). Among participants who received PENBRAYA in controlled studies, 47.4% were male, 79.4% were White, 10.2% were Black or African-American, 2.1% were Asian, and 2.6% were of other racial groups, and 21.6% were of Hispanic/Latino ethnicity.
Solicited Local and Systemic Adverse Reactions
Table 1 presents the solicited local adverse reactions and Table 2 presents the solicited systemic adverse reactions and use of antipyretic medication reported within 7 days following each dose of PENBRAYA in Study 1.
Table 1. Percentage of Participants Reporting Solicited Local Adverse Reactions Within 7 Days After PENBRAYA or Trumenba Vaccination in Study 1* Abbreviations: N = number of participants; MenACWY-CRM = meningococcal (serogroups A, C, W and Y) oligosaccharide diphtheria CRM197 conjugate vaccine; Trumenba = meningococcal serogroup B factor H binding protein. - * NCT04440163
- † Trumenba and MenACWY-CRM were administered at 0 month followed by Trumenba alone at 6 months. Local reactions were recorded only for PENBRAYA and Trumenba injection sites.
- ‡ Mild (does not interfere with activity); Moderate (interferes with activity); Severe (prevents daily activity).
- § "Any" is defined as the cumulative frequency of participants who reported a reaction as "mild", "moderate", or "severe" within 7 days of vaccination.
- ¶ Mild (2.0 to 5 cm); Moderate (>5 to 10 cm); Severe (>10 cm).
Injection Site Reactions
PENBRAYA
Trumenba + MenACWY-CRM†
Dose 1
Dose 2
Dose 1
Dose 2
N=1724-1725
%
N=1456
%
N=630-631
%
N=529
%
Pain‡
Any§
89.3
84.4
85.1
78.6
Mild
32.3
29.1
31.1
33.1
Moderate
49.4
48.8
47.7
40.3
Severe
7.5
6.5
6.3
5.3
Redness¶
Any§
25.9
23.2
19.5
14.7
Mild
8.9
7.7
7.3
6.6
Moderate
14.4
12.6
10.0
7.2
Severe
2.6
3.0
2.2
0.9
Swelling¶
Any§
25.0
24.2
21.4
14.7
Mild
10.6
10.4
8.3
6.4
Moderate
13.3
12.8
12.4
8.1
Severe
1.2
1.0
0.8
0.2
Table 2. Percentage of Participants Reporting Solicited Systemic Adverse Reactions and Use of Antipyretic Medications Within 7 Days After Each Vaccination in Study 1 Abbreviations: N = number of participants; MenACWY-CRM = meningococcal (serogroups A, C, W and Y) oligosaccharide diphtheria CRM197 conjugate vaccine; Trumenba= meningococcal serogroup B factor H binding protein. - * Trumenba and MenACWY-CRM were administered at 0 month followed by Trumenba alone at 6 months.
- † Mild (1 to 2 times in 24 hours); Moderate (>2 times in 24 hours); Severe (requires intravenous hydration).
- ‡ "Any" is defined as the cumulative frequency of participants who reported a reaction as "mild", "moderate", or "severe" within 7 days of vaccination.
- § Mild (2 to 3 loose stools in 24 hours); Moderate (4 to 5 loose stools in 24 hours); Severe (6 or more loose stools in 24 hours).
- ¶ Mild (does not interfere with activity); Moderate (interferes with activity); Severe (prevents daily activity).
Systemic Reactions
PENBRAYA
Trumenba + MenACWY-CRM*
Dose 1
Dose 2
Dose 1
Dose 2
N=1739-1740
%
N=1459
%
N=638
%
N=532
%
Fever (≥38°C)
≥38.0°C
5.9
2.4
5.8
1.5
38.0° to 38.4°C
3.7
1.9
2.0
0.4
>38.4° to 38.9°C
1.6
0.3
2.8
0.9
>38.9° to 40.0°C
0.6
0.2
0.9
0.2
>40.0°C
0.0
0.0
0.0
0.0
Vomiting†
Any‡
3.2
1.5
3.0
0.9
Mild
2.5
1.4
2.0
0.8
Moderate
0.6
0.1
0.9
0.2
Severe
0.0
0.0
0.0
0.0
Diarrhea§
Any‡
11.0
8.2
13.5
8.5
Mild
8.7
6.9
11.9
6.0
Moderate
2.0
1.4
1.6
2.4
Severe
0.3
0.0
0.0
0.0
Headache¶
Any‡
46.8
39.8
46.9
37.8
Mild
25.7
21.3
24.5
21.1
Moderate
19.2
16.8
20.4
16.2
Severe
1.9
1.7
2.0
0.6
Fatigue¶
Any‡
52.1
47.6
54.7
43.6
Mild
23.5
22.8
25.7
22.0
Moderate
25.5
21.8
25.7
19.9
Severe
3.2
2.9
3.3
1.7
Chills¶
Any‡
20.1
16.4
19.6
16.2
Mild
12.6
9.9
10.2
8.8
Moderate
6.7
6.0
7.8
5.8
Severe
0.8
0.4
1.6
1.5
Muscle pain (other than muscle pain at the injection site)¶
Any‡
25.7
22.8
27.4
22.2
Mild
13.6
10.0
13.5
10.0
Moderate
10.5
11.9
11.9
11.5
Severe
1.6
0.8
2.0
0.8
Joint pain¶
Any‡
20.2
18.3
22.6
15.6
Mild
10.7
9.6
12.9
7.9
Moderate
8.6
8.3
8.6
6.8
Severe
1.0
0.4
1.1
0.9
Use of antipyretic medication
29.5
25.1
28.1
20.5
Serious Adverse Events
In Study 1, during the 30 days after vaccination visit 1 (at 0 months), <0.1% (1/1763) of PENBRAYA and 0% (0/649) of Trumenba + MenACWY-CRM participants reported at least 1 SAE. During the 30 days after vaccination visit 2 (at 6 months), 0.1% (2/1558) of PENBRAYA and 0% (0/562) of Trumenba participants reported at least 1 SAE. None of the SAEs were determined to be related to PENBRAYA vaccination.
In Study 2, during the 30 days after vaccination visit 1 (at 0 months), 0.4% (2/543) of PENBRAYA and 0% (0/1057) of Trumenba + MenACWY-CRM participants reported at least 1 SAE. During the 30 days after vaccination visit 2 (at 6 months), 0.2% (1/486) of PENBRAYA and 0% (0/946) of Trumenba participants reported at least 1 SAE. None of the SAEs were determined to be related to PENBRAYA vaccination.
In non-controlled Study 3, no SAEs (0/294) were reported within 30 days after either vaccination (0, 12 months).
6.2 Postmarketing Experience
The postmarketing safety experience with Trumenba and a non-U.S.-licensed Meningococcal Groups A, C, W, and Y polysaccharide tetanus toxoid (TT) conjugate vaccine (MenACWY-TT vaccine; Pfizer Inc.) is relevant to PENBRAYA since PENBRAYA includes the same group A, C, W, and Y TT-conjugated polysaccharide components and MenB recombinant protein components. Because these events are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship to vaccination. The following adverse reactions have been spontaneously reported during postmarketing use of Trumenba and MenACWY-TT and may also be seen in postmarketing experience with PENBRAYA.
Immune System Disorders: allergic reactions, including anaphylaxis
Nervous System: syncope (fainting)
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in individuals exposed to PENBRAYA during pregnancy. Individuals who received PENBRAYA during pregnancy are encouraged to contact, or have their healthcare provider contact, 1-877-390-2953 to enroll in or obtain information about the registry.
Risk Summary
All pregnancies have a risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
There are no clinical studies of PENBRAYA in pregnant individuals. Available human data on PENBRAYA administered to pregnant individuals are insufficient to inform vaccine-associated risks in pregnancy.
There were no developmental toxicity studies performed with PENBRAYA.
8.2 Lactation
Risk Summary
There are no data available to assess the effects of PENBRAYA on the breastfed infant or on milk production/excretion. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for PENBRAYA and any potential adverse effects on the breastfed child from PENBRAYA or from the underlying maternal condition. For preventive vaccines, the underlying maternal condition is susceptibility to disease prevented by the vaccine.
8.4 Pediatric Use
The safety and effectiveness of PENBRAYA have not been established in individuals <10 years of age. In a clinical study, 90% of infants younger than 12 months of age who were vaccinated with a reduced dosage formulation of Trumenba had fever. PENBRAYA contains the same MenB component, in the same quantity, as Trumenba.
-
11 DESCRIPTION
PENBRAYA (Meningococcal Groups A, B, C, W, and Y Vaccine) is a suspension for intramuscular injection. PENBRAYA is supplied as a sterile Lyophilized MenACWY Component to be reconstituted with the sterile MenB Component.
The Lyophilized MenACWY Component consists of N. meningitidis serogroups A, C, W, and Y polysaccharides individually conjugated to TT. The polysaccharide for each group is grown in media containing dextrose, salt, and yeast extract, then purified by precipitation and filtration. The TT is produced by fermentation of Clostridium tetani in dextrose, salts, and tryptone N1 peptone followed by formalin detoxification, then purified by a series of physicochemical steps. The serogroups A and C polysaccharides are individually microfluidized, activated with 1-cyano-4(dimethylamino)-pyridinium tetrafluoroborate (CDAP), derivatized with adipic acid dihydrazide (ADH), and then conjugated with TT in the presence of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide) (EDAC). The serogroups W and Y polysaccharides are individually microfluidized, activated with CDAP, and then conjugated with TT. The conjugates are purified by a series of physicochemical steps then sterile filtered. Trometamol/sucrose buffer is added, and the MenACWY-TT solution is lyophilized.
The MenB Component is a sterile suspension composed of 2 recombinant lipidated factor H binding protein (fHbp) variants from N. meningitidis serogroup B, 1 from fHbp subfamily A and 1 from fHbp subfamily B (A05 and B01, respectively). The proteins are individually produced in Escherichia coli. Production strains are grown to a specific density in chemically defined fermentation growth media without antibiotics or animal-derived components. The recombinant proteins are extracted from the production strains and purified through a series of column chromatography steps. Polysorbate 80 (PS80) is added and is present in the MenB Component.
Each approximately 0.5 mL dose of PENBRAYA contains N. meningitidis serogroup A, C, W, and Y polysaccharide (5 mcg each; 20 mcg total) conjugated to tetanus toxoid (44 mcg tetanus toxoid), 2 recombinant lipidated factor H binding protein variants from N. meningitidis serogroup B (60 mcg each; total of 120 mcg protein), L-histidine (0.78 mg) , trometamol (0.097 mg), sucrose (28 mg), aluminum phosphate (0.25 mg aluminum), sodium chloride (4.65 mg), and PS80 80 (0.018 mg) at pH 6.0.
PENBRAYA does not contain any preservatives.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Protection against invasive meningococcal disease is conferred mainly by complement-mediated antibody-dependent killing of N. meningitidis.1 Vaccination with PENBRAYA induces the production of bactericidal antibodies specific to the capsular polysaccharides of N. meningitidis serogroups A, C, W, and Y and to fHbp subfamily A and B variants of N. meningitidis group B. The susceptibility of group B meningococci to bactericidal antibody is dependent upon both the antigenic similarity of the fHbp subfamily A or subfamily B vaccine antigen to the fHbp protein expressed by the bacterial strain and the amount of fHbp expressed at the bacterial surface.2
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
The effectiveness of PENBRAYA was assessed in Study 1 by measuring antibodies with assays that used human complement to assess serum bactericidal activity (hSBA). For serogroups A, C, W, and Y, one strain was utilized per group. For serogroup B, four meningococcal serogroup B strains expressing different fHbp variants that represent the A and B subfamilies of meningococcal serogroup B strains causing invasive disease in the U.S. and Europe were utilized. The proportions of subjects with a 4-fold or greater increase in hSBA titer for each strain, and the proportion of subjects with a titer greater than or equal to the lower limit of quantitation (LLOQ) of the assay for all four serogroup B strains (composite response) were assessed.
14.1 Immunogenicity
Study 1 was a Phase 3, randomized, active-controlled, observer-blinded, multicenter study in which participants 10 through 25 years of age in the U.S. and Europe received PENBRAYA at 0 and 6 months or Trumenba at 0 and 6 months and MenACWY-CRM at 0 months. All participants were MenB vaccine-naïve. Both MenACWY conjugate vaccine -naïve and MenACWY conjugate vaccine-exposed participants were part of the study.
Seroresponse for serogroups A, B, C, W, and Y and composite response for serogroup B following 2 doses of PENBRAYA in Study 1 are presented in Tables 3 and 4.
Among both ACWY-naïve and ACWY-exposed participants, seroresponse rates to serogroups A, C, W, and Y following 2 doses of PENBRAYA were demonstrated to be non-inferior to seroresponse rates following a single dose of MenACWY-CRM. Seroresponse and composite response rates to serogroup B primary strains among participants who received 2 doses of PENBRAYA were demonstrated to be non-inferior to seroresponse and composite response rates following 2 doses of Trumenba.
Table 3. Percentage of Subjects Achieving Seroresponses 1 Month after Receiving 2 Doses of PENBRAYA (0 and 6 Months) versus 1 Month after Single Dose of MenACWY-CRM for Serogroups A, C, W, and Y (Study 1)*,† Abbreviations: CI = confidence interval; hSBA = serum bactericidal assay using human complement; LLOQ = lower limit of quantitation; LOD = limit of detection; MenACWY-CRM = meningococcal (serogroups A, C, W and Y) oligosaccharide diphtheria CRM197 conjugate vaccine; Trumenba= meningococcal serogroup B factor H binding protein. Note: The LLOQ is an hSBA titer = 1:16 for A22 and 1:8 for A56, B24, and B44 and serogroups A, C, W, and Y. Note: Seroresponse is defined as the 4-fold increase as follows: (1) For participants with a baseline hSBA titer <1:4 (LOD), a 4-fold response was defined as an hSBA titer ≥1:16. (2) For participants with a baseline hSBA titer ≥ LOD and < LLOQ, a response is defined as an hSBA titer ≥4 times the LLOQ. (3) For participants with a baseline hSBA titer ≥ LLOQ, a response is defined as an hSBA titer ≥4 times the baseline titer. - * Evaluable immunogenicity populations.
- † NCT04440163
- ‡ Non-inferiority was demonstrated (using 10% margin) post-vaccination by assessing the difference between vaccination serogroups.
Serogroup
PENBRAYA
%
Trumenba + MenACWY-CRM
%
PENBRAYA – MenACWY-CRM
Difference‡
N=439-451 (Naïve)
N=376-387 (Exposed)
N=244-254 (Naïve)
N=222-227 (Exposed)
Difference %
(95% CI)
A
ACWY-naïve
97.8
95.3
2.5
(-0.2, 6.0)
ACWY-exposed
93.8
96.9
-3.2
(-6.5, 0.5)
C
ACWY-naïve
93.3
52.4
41.0
(34.4, 47.5)
ACWY-exposed
93.8
94.7
-0.9
(-4.6, 3.3)
W
ACWY- naïve
97.3
73.0
24.3
(18.8, 30.4)
ACWY-exposed
97.1
96.4
0.7
(-2.2, 4.3)
Y
ACWY- naïve
94.4
70.6
23.8
(18.0, 30.1)
ACWY-exposed
93.0
93.7
-0.7
(-4.6, 3.8)
Table 4. Percentage of Participants Achieving Seroresponses and Composite Response 1 Month after Receiving 2 Doses of PENBRAYA (0 and 6 Months) versus 2 Doses of Trumenba (0 and 6 Months) For Serogroup B (Study 1)*,† Abbreviations: CI = confidence interval; hSBA = serum bactericidal assay using human complement; LLOQ = lower limit of quantitation; LOD = limit of detection; MenACWY-CRM = meningococcal (serogroups A, C, W and Y) oligosaccharide diphtheria CRM197 conjugate vaccine; Trumenba= meningococcal serogroup B factor H binding protein. Note: The LLOQ is an hSBA titer = 1:16 for A22 and 1:8 for A56, B24, and B44 and serogroups A, C, W, and Y. Note: Seroresponse is defined as the 4-fold increase as follows: (1) For participants with a baseline hSBA titer <1:4 (LOD), a 4-fold response was defined as an hSBA titer ≥1:16. (2) For participants with a baseline hSBA titer ≥ LOD and < LLOQ, a response is defined as an hSBA titer ≥4 times the LLOQ. (3) For participants with a baseline hSBA titer ≥ LLOQ, a response is defined as an hSBA titer ≥4 times the baseline titer. - * Evaluable immunogenicity populations.
- † NCT04440163
- ‡ Non-inferiority was demonstrated (using 10% margin) post-vaccination by assessing the difference between vaccination serogroups.
- § Composite response = hSBA ≥ LLOQ for all 4 primary meningococcal B strains.
PENBRAYA
%
Trumenba + MenACWY-CRM
%
PENBRAYA – Trumenba
Difference
Serogroup B Variant
N=755-845
N=383-419
Difference %
(95% CI)
Seroresponse‡
A22
83.0
79.0
4.0
(-0.7, 8.9)
A56
95.9
94.5
1.4
(-1.0, 4.3)
B24
68.1
57.2
10.9
(5.2, 16.6)
B44
86.5
79.2
7.3
(2.9, 11.9)
Composite§
Pre-Dose 1
1.2
2.0
-
Post-Dose 2
78.3
68.7
9.6
(4.2, 15.2)
-
15 REFERENCES
- 1. Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. (1969);129:1307–1326.
- 2. Wang X, et al. Prevalence and genetic diversity of candidate vaccine antigens among invasive Neisseria meningitidis isolates in the U.S. Vaccine 2011; 29:4739-4744.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
PENBRAYA is supplied in a kit that includes a vial of Lyophilized MenACWY Component (NDC: 0069-0271-01), a prefilled syringe containing MenB Component (NDC: 0069-0332-01) and a vial adapter. The Lyophilized MenACWY Component is reconstituted with the MenB Component to form a single dose of PENBRAYA.
PENBRAYA is supplied in cartons of 1, 5, and 10 kits.
Carton: 1 kit
0069-0600-01
Carton: 5 kits
0069-0600-05
Carton: 10 kits
0069-0600-10
The vial stopper and the syringe tip cap and plunger stopper are not made with natural rubber latex.
16.2 Storage Before Reconstitution
Store refrigerated at 2°C to 8°C (36°F to 46°F) in the original carton. During storage, a white deposit and clear supernatant may be observed in the prefilled syringe containing the MenB Component. Store the carton horizontally to minimize the time necessary to resuspend the MenB Component.
Do not freeze. Discard if the carton has been frozen.
-
17 PATIENT COUNSELING INFORMATION
Prior to administration of PENBRAYA:
- Inform vaccine recipient of the potential benefits and risks of vaccination with PENBRAYA.
- Advise vaccine recipient to report any adverse events to their healthcare provider or to the Vaccine Adverse Event Reporting System at 1-800-822-7967 and https://vaers.hhs.gov/.
This product’s labeling may have been updated. For the most recent prescribing information, please visit https://fda.report/dailymed/.
Manufactured by:
Pfizer Ireland Pharmaceuticals
Ringaskiddy, Cork, IrelandUS License No. 2060
LAB-1512-4.0
-
PRINCIPAL DISPLAY PANEL - 1 Vial/Syringe Kit Carton
NDC: 0069-0600-01
Rx onlyNOTICE: Lyophilized MenACWY
Component and MenB Component
must be combined before use.Meningococcal Groups
A, B, C, W, and Y Vaccine
PENBRAYA®For Intramuscular Use Only
Contents: 1 kit. The kit provides a single dose of PENBRAYA®
Each kit contains:
1 vial of Lyophilized MenACWY Component
1 syringe of MenB Component
1 vial adapterAfter reconstitution, a single dose of PENBRAYA is
approximately 0.5 mL.For use in individuals 10 through 25 years of age
Pfizer
-
PRINCIPAL DISPLAY PANEL - 5 Vial/Syringe Kit Carton
NDC: 0069-0600-05
Rx onlyNOTICE: Lyophilized MenACWY Component
and MenB Component must be combined
before use.Meningococcal Groups
A, B, C, W, and Y Vaccine
PENBRAYA®For Intramuscular Use Only
Contents: 5 kits. Each kit provides a
single dose of PENBRAYA®
Each kit contains:
1 vial of Lyophilized MenACWY
Component
1 syringe of MenB Component
1 vial adapterAfter reconstitution, a single dose of
PENBRAYA is approximately 0.5 mL.For use in individuals 10 through
25 years of agePfizer
-
PRINCIPAL DISPLAY PANEL - 10 Vial/Syringe Kit Carton
NDC: 0069-0600-10
Rx onlyNOTICE: Lyophilized MenACWY Component and
MenB Component must be combined before use.Meningococcal Groups
A, B, C, W, and Y Vaccine
PENBRAYA®For Intramuscular Use Only
Contents: 10 kits. Each kit provides a single dose of
PENBRAYA®
Each kit contains:
1 vial of Lyophilized MenACWY Component
1 syringe of MenB Component
1 vial adapterAfter reconstitution, a single dose of PENBRAYA is
approximately 0.5 mL.For use in individuals 10 through 25 years of age
Pfizer
-
PRINCIPAL DISPLAY PANEL - 1 Vial Label
Lyophilized
MenACWY ComponentNDC: 0069-0271-01
NOT TO BE USED ALONE
Reconstitute with MenB Component
to form Meningococcal Groups A, B,
C, W, and Y Vaccine (PENBRAYA®)Single dose of approximately
0.5 mL after reconstitutionRx only
Pfizer Ireland
US Lic. No. 2060 -
PRINCIPAL DISPLAY PANEL - 1 Syringe Label
NDC: 0069-0332-01
Rx onlyMenB Component
Add to 1 vial of Lyophilized
MenACWY Component to form
Meningococcal
Groups A, B, C, W,
and Y Vaccine
(PENBRAYA®)NOT TO BE USED ALONE
PAA234031
Lot:
Exp:MenB Component
NOT TO BE USED ALONE
SHAKE WELL
Add to 1 vial of Lyophilized
MenACWY Component to form
Meningococcal
Groups A, B, C, W, and Y Vaccine
(PENBRAYA®)Pfizer Ireland
US Lic. No. 2060 -
INGREDIENTS AND APPEARANCE
PENBRAYA
meningococcal groups a, b, c, w, and y vaccine kitProduct Information Product Type VACCINE Item Code (Source) NDC: 0069-0600 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0069-0600-01 1 in 1 CARTON 1 NDC: 0069-0600-99 1 in 1 KIT; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) 2 NDC: 0069-0600-05 5 in 1 CARTON 2 NDC: 0069-0600-99 1 in 1 KIT; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) 3 NDC: 0069-0600-10 10 in 1 CARTON 3 NDC: 0069-0600-99 1 in 1 KIT; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, GLASS 0.5 mL Part 2 1 SYRINGE 0.5 mL Part 1 of 2 LYOPHILIZED MENACWY COMPONENT
lyophilized menacwy component injection, powder, lyophilized, for suspensionProduct Information Item Code (Source) NDC: 0069-0271 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NEISSERIA MENINGITIDIS GROUP A CAPSULAR POLYSACCHARIDE TETANUS TOXOID CONJUGATE ANTIGEN (UNII: T4GYX3110D) (NEISSERIA MENINGITIDIS GROUP A CAPSULAR POLYSACCHARIDE TETANUS TOXOID CONJUGATE ANTIGEN - UNII:T4GYX3110D) NEISSERIA MENINGITIDIS GROUP A CAPSULAR POLYSACCHARIDE TETANUS TOXOID CONJUGATE ANTIGEN 5 ug in 0.5 mL NEISSERIA MENINGITIDIS GROUP C CAPSULAR POLYSACCHARIDE TETANUS TOXOID CONJUGATE ANTIGEN (UNII: ZT89E5A103) (NEISSERIA MENINGITIDIS GROUP C CAPSULAR POLYSACCHARIDE TETANUS TOXOID CONJUGATE ANTIGEN - UNII:ZT89E5A103) NEISSERIA MENINGITIDIS GROUP C CAPSULAR POLYSACCHARIDE TETANUS TOXOID CONJUGATE ANTIGEN 5 ug in 0.5 mL NEISSERIA MENINGITIDIS GROUP W-135 CAPSULAR POLYSACCHARIDE TETANUS TOXOID CONJUGATE ANTIGEN (UNII: L77OK410KW) (NEISSERIA MENINGITIDIS GROUP W-135 CAPSULAR POLYSACCHARIDE TETANUS TOXOID CONJUGATE ANTIGEN - UNII:L77OK410KW) NEISSERIA MENINGITIDIS GROUP W-135 CAPSULAR POLYSACCHARIDE TETANUS TOXOID CONJUGATE ANTIGEN 5 ug in 0.5 mL NEISSERIA MENINGITIDIS GROUP Y CAPSULAR POLYSACCHARIDE TETANUS TOXOID CONJUGATE ANTIGEN (UNII: 4WAN8PQK15) (NEISSERIA MENINGITIDIS GROUP Y CAPSULAR POLYSACCHARIDE TETANUS TOXOID CONJUGATE ANTIGEN - UNII:4WAN8PQK15) NEISSERIA MENINGITIDIS GROUP Y CAPSULAR POLYSACCHARIDE TETANUS TOXOID CONJUGATE ANTIGEN 5 ug in 0.5 mL Inactive Ingredients Ingredient Name Strength CLOSTRIDIUM TETANI TOXOID ANTIGEN (FORMALDEHYDE INACTIVATED) (UNII: K3W1N8YP13) 44 ug in 0.5 mL TROMETHAMINE (UNII: 023C2WHX2V) 97 ug in 0.5 mL SUCROSE (UNII: C151H8M554) 28 mg in 0.5 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0069-0271-01 0.5 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125770 11/27/2023 Part 2 of 2 MENB COMPONENT
menb component suspensionProduct Information Item Code (Source) NDC: 0069-0332 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NEISSERIA MENINGITIDIS GROUP B RECOMBINANT LP2086 A05 PROTEIN VARIANT ANTIGEN (UNII: 583WCD0IZI) (NEISSERIA MENINGITIDIS GROUP B RECOMBINANT LP2086 A05 PROTEIN VARIANT ANTIGEN - UNII:583WCD0IZI) NEISSERIA MENINGITIDIS GROUP B RECOMBINANT LP2086 A05 PROTEIN VARIANT ANTIGEN 0.06 mg in 0.5 mL NEISSERIA MENINGITIDIS GROUP B RECOMBINANT LP2086 B01 PROTEIN VARIANT ANTIGEN (UNII: 7MBD4K530D) (NEISSERIA MENINGITIDIS GROUP B RECOMBINANT LP2086 B01 PROTEIN VARIANT ANTIGEN - UNII:7MBD4K530D) NEISSERIA MENINGITIDIS GROUP B RECOMBINANT LP2086 B01 PROTEIN VARIANT ANTIGEN 0.06 mg in 0.5 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 4.38 mg in 0.5 mL HISTIDINE (UNII: 4QD397987E) 0.78 mg in 0.5 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 0.018 mg in 0.5 mL ALUMINUM PHOSPHATE (UNII: F92V3S521O) 0.25 mg in 0.5 mL WATER (UNII: 059QF0KO0R) 498 mg in 0.5 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0069-0332-01 0.5 mL in 1 SYRINGE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125770 11/27/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125770 11/27/2023 Labeler - Pfizer Laboratories Div Pfizer Inc (134489525) Establishment Name Address ID/FEI Business Operations Pfizer Health AB 354433591 ANALYSIS(0069-0600) , API MANUFACTURE(0069-0600) Establishment Name Address ID/FEI Business Operations Pfizer Inc 004954111 API MANUFACTURE(0069-0600) , ANALYSIS(0069-0600) Establishment Name Address ID/FEI Business Operations Wyeth Pharmaceutical Division of Wyeth Holdings LLC 883534067 ANALYSIS(0069-0600) , API MANUFACTURE(0069-0600) Establishment Name Address ID/FEI Business Operations Pfizer Ireland Pharmaceuticals Unlimited Company 985586408 ANALYSIS(0069-0600) , API MANUFACTURE(0069-0600) , MANUFACTURE(0069-0600) Establishment Name Address ID/FEI Business Operations Pfizer Manufacturing Austria GmbH 300453240 ANALYSIS(0069-0600) Establishment Name Address ID/FEI Business Operations Pfizer Manufacturing Belgium NV 370156507 ANALYSIS(0069-0600) , MANUFACTURE(0069-0600) , PACK(0069-0600) , LABEL(0069-0600)
Trademark Results [PENBRAYA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 PENBRAYA 97621374 not registered Live/Pending |
Pfizer Inc. 2022-10-06 |
 PENBRAYA 88592990 not registered Live/Pending |
Pfizer Inc. 2019-08-26 |
 PENBRAYA 86962914 not registered Dead/Abandoned |
Pfizer Inc. 2016-04-04 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.