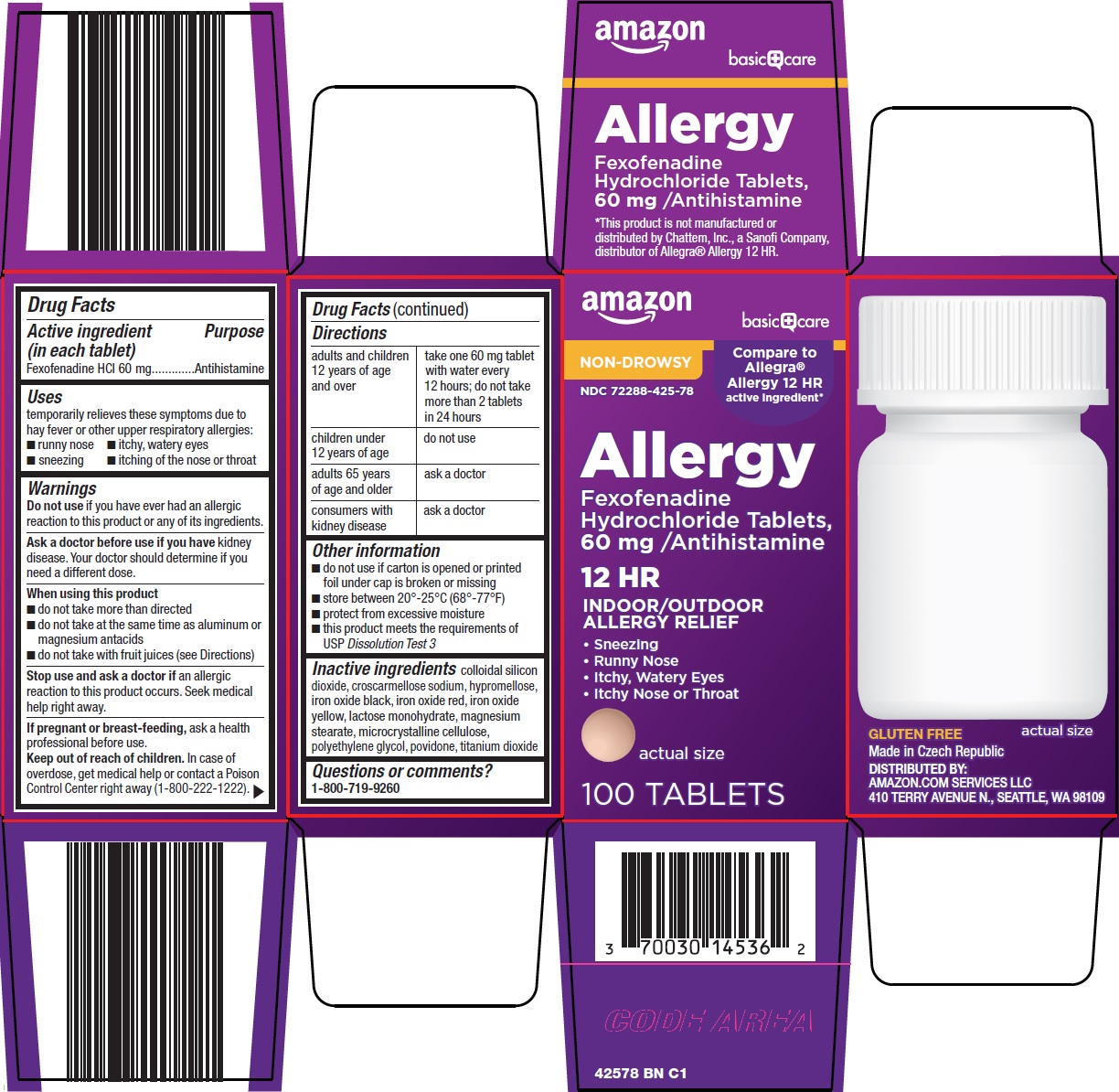
Amazon Allergy Drug Facts
basic care allergy by
Drug Labeling and Warnings
basic care allergy by is a Otc medication manufactured, distributed, or labeled by Amazon.com Services LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
BASIC CARE ALLERGY- fexofenadine hcl tablet, film coated
Amazon.com Services LLC
----------
Amazon Allergy Drug Facts
Uses
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- itchy, watery eyes
- sneezing
- itching of the nose or throat
Warnings
Ask a doctor before use if you have
kidney disease. Your doctor should determine if you need a different dose.
When using this product
- do not take more than directed
- do not take at the same time as aluminum or magnesium antacids
- do not take with fruit juices (see Directions)
Directions
|
adults and children 12 years of age and over |
take one 60 mg tablet with water every 12 hours; do not take more than 2 tablets in 24 hours |
|
children under 12 years of age |
do not use |
|
adults 65 years of age and older |
ask a doctor |
|
consumers with kidney disease |
ask a doctor |
Other information
- do not use if printed blister unit is broken or torn
- store between 20°-25°C (68°-77°F)
- protect from excessive moisture
- this product meets the requirements of USP Dissolution Test 3
Inactive ingredients
colloidal silicon dioxide, croscarmellose sodium, hypromellose, iron oxide black, iron oxide red, iron oxide yellow, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, povidone, titanium dioxide
Package/Label Principal Display Panel
amazon
basic care
NON-DROWSY
Compare to Allegra® Allergy 12 HR
active ingredient
Allergy
Fexofenadine Hydrochloride Tablets, 60 mg /Antihistamine
12 HR
INDOOR/OUTDOOR ALLERGY RELIEF
Sneezing
Runny Nose
Itchy, Watery Eyes
Itchy Nose or Throat
actual size
12 TABLETS
| BASIC CARE ALLERGY
fexofenadine hcl tablet, film coated |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Amazon.com Services LLC (128990418) |