LIDOCAINE HYDROCHLORIDE AND HYDROCORTISONE ACETATE gel
Lidocaine Hydrochloride and Hydrocortisone Acetate by
Drug Labeling and Warnings
Lidocaine Hydrochloride and Hydrocortisone Acetate by is a Prescription medication manufactured, distributed, or labeled by Seton Pharmaceuticals. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- DESCRIPTION:
- ACTIVE INGREDIENTS:
- INACTIVE INGREDIENTS:
-
CLINICAL PHARMACOLOGY:
MECHANISM OF ACTION:
Product releases lidocaine to stabilize the neuronal membrane by inhibiting the ionic fluxes required for initiation and conduction of impulses, thereby effecting local anesthetic action. Hydrocortisone acetate provides relief of inflammatory and pruritic manifestations of corticosteroid responsive dermatoses. Lidocaine is chemically designated as acetamide, 2-(diethylamino)-N-(2,6-dimethylphenyl), and has the following structure:
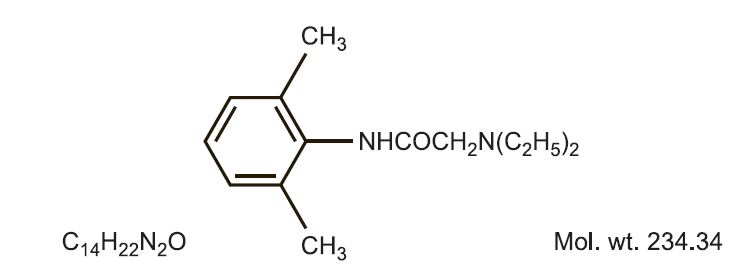
Hydrocortisone acetate has a chemical name pregn-4-ene-3, 20-dione, 21-(acetyloxy)-11,17- dihydroxy-(11ß)-. It has the following structural formula:
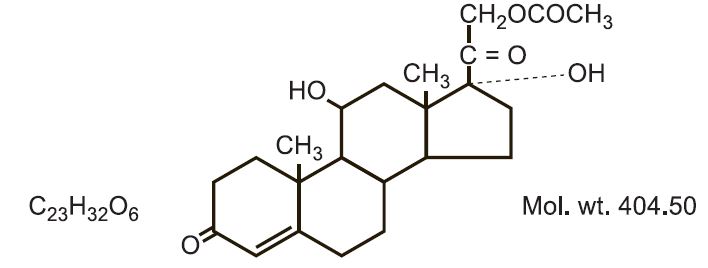
-
CONTRAINDICATIONS:
Product should not be used in patients with a history of sensitivity to any of its ingredients or adverse reactions to lidocaine or amide anesthetics, which usually do not cross-react with “caine” ester type anesthetics. If excessive irritation and significant worsening occur, discontinue use and seek the advice of your physician. Product and topical lidocaine should be used cautiously in those with impaired liver function, as well as the very ill or very elderly and those with significant liver disease. Product should be used with caution on patients receiving antiarrhythmic drugs of Class I since the adverse effects are additive and generally synergistic. These products are contraindicated for tuberculous or fungal lesions or skin vaccinia, varicella and acute herpes simplex. Topical corticosteroids are contraindicated in those patients with a history of hypersensitivity to any of the components of the preparation.
-
WARNINGS:
For external use only. Not for ophthalmic use.
Product, applicators and moist wipes could harm small children if chewed or swallowed.
Keep product, moist wipes and applicators out of the reach of children.
Methemoglobinemia
Cases of methemoglobinemia have been reported in association with local anesthetic use. Although all patients are at risk for methemoglobinemia, patients with glucose-6-phosphate dehydrogenase deficiency, congenital or idiopathic methemoglobinemia, cardiac or pulmonary compromise, infants under 6 months of age, and concurrent exposure to oxidizing agents or their metabolites are more susceptible to developing clinical manifestations of the condition. If local anesthetics must be used in these patients, close monitoring for symptoms and signs of methemoglobinemia is recommended.
Signs and symptoms of methemoglobinemia may occur immediately or may be delayed some hours after exposure and are characterized by a cyanotic skin discoloration and abnormal coloration of the blood. Methemoglobin levels may continue to rise; therefore, immediate treatment is required to avert more serious central nervous system and cardiovascular adverse effects, including seizures, coma, arrhythmias, and death. Discontinue [the use of this product] and any other oxidizing agents. Depending on the severity of the symptoms, patients may respond to supportive care, i.e., oxygen therapy, hydration. More severe symptoms may require treatment with methylene blue, exchange transfusion, or hyperbaric oxygen.
-
DRUG INTERACTIONS:
Patients that are administered local anesthetics may be at increased risk of developing methemoglobinemia when concurrently exposed to the following oxidizing agents:
Class Examples Nitrates/Nitrites nitroglycerin, nitroprusside, nitric oxide, nitrous oxide Local anesthetics benzocaine, lidocaine, bupivacaine, mepivacaine, tetracaine, prilocaine, procaine, articaine, ropivacaine Antineoplastic agents cyclophosphamide, flutamide, rasburicase, ifosfamide, hydroxyurea Antibiotics dapsone, sulfonamides, nitrofurantoin, para-aminosalicylic acid Antimalarials chloroquine, primaquine Anticonvulsants phenytoin, sodium valproate, phenobarbital Other drugs acetaminophen, metoclopramide, sulfa drugs (i.e., sulfasalazine), quinine -
PATIENT COUNSELING INFORMATION:
Inform patients that use of local anesthetics may cause methemoglobinemia, a serious condition that must be treated promptly. Advise patients or caregivers to stop use and seek immediate medical attention if they or someone in their care experience the following signs or symptoms: pale, gray, or blue colored skin (cyanosis); headache; rapid heart rate; shortness of breath; lightheadedness; or fatigue.
Topical formulations of lidocaine may be absorbed to a greater extent through mucous membranes and abraded, fissured or irritated skin than through intact skin. Product should not be ingested or applied into the mouth, inside of the nose or in the eyes. Product should not be used in the ears. Any situation where lidocaine penetrates beyond the tympanic membrane into the middle ear is contraindicated because of ototoxicity associated with lidocaine observed in animals when instilled in the middle ear. Product should not come into contact with the eye or be applied into the eye because of the risk of severe eye irritation and the loss of eye surface sensation which reduces protective reflexes and can lead to corneal irritation and possibly abrasion. If eye contact occurs, rinse out the eye immediately with saline or water and protect the eye surface until sensation is restored.
-
PRECAUTIONS:
If irritation or sensitivity occurs or infection appears, discontinue use and institute appropriate therapy. If extensive areas are treated, the possibility of systemic absorption exists. Systemic absorption of topical steroids has produced reversible hypothalamic pituitary-adrenal (HPA) axis suppression, manifestations of Cushing’s syndrome, hyperglycemia, and glycosuria in some patients. Conditions which augment systemic absorption include the application of the more potent steroids, use over large surface areas, prolonged use, and the addition of occlusive dressings. Therefore, patients receiving a large dose of potent topical steroids applied to a large surface area, or under an occlusive dressing, should be evaluated periodically for evidence of HPA axis suppression. If noted, an attempt should be made to withdraw the drug to reduce the frequency of application, or to substitute a less potent steroid.
Recovery of the HPA axis function is generally prompt and complete upon discontinuation of the drug. Infrequently, signs and symptoms of steroid withdrawal may occur, requiring supplemental systemic corticosteroids. Children may absorb proportionately larger amounts of topical cortico-steroids and thus be more susceptible to systemic toxicity. If irritation develops, topical steroids should be discontinued and appropriate therapy instituted. In the presence of dermatological infections, the use of an appropriate antifungal or antibacterial agent should be instituted. If a favorable response does not occur promptly, the corticosteroid should be discontinued until the infection has been adequately controlled.
-
CARCINOGENESIS, MUTAGENESIS, AND IMPAIRMENT OF FERTILITY:
Long-term animal studies have not been performed to evaluate the carcinogenic potential or the effect on fertility of topical corticosteroids. Studies to determine mutagenicity with prednisolone and hydrocortisone have revealed negative results. Studies of lidocaine in animals to evaluate the carcinogenic and mutagenic potential of the effect on fertility have not been conducted.
-
USE IN PREGNANCY:
Teratogenic Effects:
Pregnancy Category C. Reproduction studies have been performed for lidocaine in rats at doses up to 6.6 times the human dose and have revealed no evidence of harm to the fetus caused by lidocaine. There are, however, no adequate and well-controlled studies in pregnant women. Animal reproduction studies are not always predictive of human response. General consideration should be given to this fact before administering lidocaine to women of childbearing potential, especially during early pregnancy when maximum organogenesis takes place. Corticosteroids are generally teratogenic in laboratory animals when administered systemically at relatively low dosage levels. The more potent corticosteroids have been shown to be teratogenic after dermal application in laboratory animals. There are no adequate and well controlled studies in pregnant women on teratogenic effects from topically applied corticosteroids. Therefore, topical corticosteroids should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Drugs of this class should not be used extensively on pregnant patients, in large amounts, or for prolonged periods of time.
- NURSING MOTHERS:
- PEDIATRIC USE:
- ADVERSE REACTIONS:
-
DOSAGE AND ADMINISTRATION:
Apply product to the affected area(s) twice daily or as directed by a physician. Product should not be used in excess of recommendations or for prolonged use in the anal canal. If the condition does not respond to repeated courses of product or should worsen, discontinue use and seek the advice of your physician.
Patient Directions for Rectal Administration:
Remove moist wipe from box, tear open the package, gently clean the affected area and discard moist wipe. (Note: The moist wipe does not contain any of the prescribed active ingredients.) The cap and foil seal should be removed from the tube and the applicator tip firmly screwed onto the end of the tube and tightened. (Do not over tighten.) While holding the tube, gently squeeze the tube until a small amount of cream/gel comes out of the applicator openings. This will lubricate the applicator tip. Gently insert the applicator tip into anal area. Continue squeezing the body of the tube as you move it around the areas of discomfort, and lastly, around and in the anal opening (if directed by physician). Do not completely insert the applicator and tube into the anus or insert deep into the rectum. Do not insert a loose applicator tip into the anus or rectum. Once application is completed, the tube and applicator tip should be gently removed and discarded.
-
HOW SUPPLIED:
Lidocaine 3% - Hydrocortisone 2.5% Gel Kit
20 Count Kit, NDC: 13925-164-20
Containing: 20 single use ¼ oz (7g) Tubes (NDC: 13925-164-07) of Lidocaine 3% -
Hydrocortisone 2.5% Gel (a white gel), 20 Applicators and 20 Moist Wipes
- STORAGE AND HANDLING SECTION:
-
SPL UNCLASSIFIED SECTION
All prescriptions using this product shall be pursuant to state statutes as applicable.This is not an Orange Book product. This product may be administered only under a physician’s supervision. There are no implied or explicit claims on the therapeutic equivalence.
Marketed by:
Seton Pharmaceuticals
Manasquan, NJ 08736
1-800-510-3401
Iss. 01/19
1900009
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL:
-
INGREDIENTS AND APPEARANCE
LIDOCAINE HYDROCHLORIDE AND HYDROCORTISONE ACETATE
lidocaine hydrochloride and hydrocortisone acetate gelProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 13925-164 Route of Administration RECTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 30 mg in 1 g HYDROCORTISONE ACETATE (UNII: 3X7931PO74) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE ACETATE 25 mg in 1 g Inactive Ingredients Ingredient Name Strength ALUMINUM SULFATE (UNII: 34S289N54E) CALCIUM ACETATE (UNII: Y882YXF34X) CETYL ALCOHOL (UNII: 936JST6JCN) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) PEG-100 STEARATE (UNII: YD01N1999R) METHYLPARABEN (UNII: A2I8C7HI9T) MINERAL OIL (UNII: T5L8T28FGP) PEG-150 DISTEARATE (UNII: 6F36Q0I0AC) PETROLATUM (UNII: 4T6H12BN9U) POLYCARBOPHIL (UNII: W25LM17A4W) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SODIUM CITRATE (UNII: 1Q73Q2JULR) SODIUM HYDROXIDE (UNII: 55X04QC32I) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) XANTHAN GUM (UNII: TTV12P4NEE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 13925-164-20 20 in 1 KIT 06/17/2011 1 NDC: 13925-164-07 7 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 06/17/2011 Labeler - Seton Pharmaceuticals (828898002)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
