CETIRIZINE HYDROCHLORIDE- cetirizine hydrochloride tablet, chewable
cetirizine hydrochloride by
Drug Labeling and Warnings
cetirizine hydrochloride by is a Otc medication manufactured, distributed, or labeled by Jubilant Cadista Pharmaceuticals Inc., Jubilant Generics Limited, Jubilant Generics Limited, Roorkee. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active Ingredient (in each chewable tablet)
- Purpose
- Uses:
-
Warnings:
Severe Allergy Warning: Get emergency help immediately if you have hives along with any of the following symptoms:
-
trouble swallowing
-
swelling of tongue
-
trouble speaking
-
wheezing or problems breathing
-
dizziness or loss of conciousness
-
swelling in or around mouth
-
drooling
These symptoms may be signs of anaphylactic shock. This condition can be life threatening if not treated by health professional immediately. Symptoms of anaphylactic shock may occur when hives first appear or up to a few hours later.
Not a Substitute for Epinephrine. If your doctor has prescribed an epinephrine injector for "anaphylaxis" or severe allergy symptoms that could occur with your hives, never use this product as a substitute for the epinephrine injector. If you have been prescribed an epinephrine injector, you should carry it with you at all times.
-
-
Do not use
-
to prevent hives from any known cause such as:
-
foods
-
insect stings
-
medicines
-
latex or rubber gloves
because this product will not stop hives from occuring. Avoiding the cause of your hives is the only way to prevent them. Hives can sometimes be serious.
If you do not know the cause of your hives, see your doctor for a medical exam.
Your doctor may be able to help you find a cause.● if you have ever had an allergic reaction to this product or any of its ingredients or to an antihistamine containing hydroxyzine.
-
- Ask a doctor before use if you have
- Ask a doctor or pharmacist before use if you are taking transquillizers or sedatives
- Stop use and ask doctor if
- If pregnant or breast-feeding:
- Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away at (1-800-222-1222).
-
Directions:
● may be taken with and without water
For Cetirizine Hydrochloride Chewable Tablets 5 mg
adults and children 6 years and over
1 to 2 tablets once daily depending upon severity of symptoms; do not take more than 2 tablets in 24 hours
adults 65 years and over
1 tablet once a day; do not take more than 1 tablet in 24 hours
children under 6 years of age
ask a doctor
consumers with liver or kidney disease
ask a doctor
Cetirizine Hydrochloride Chewable Tablets 10 mg
adults and children 6 years and over
one 10 mg tablet once daily; do not take more than one 10 mg tablet in 24 hours. A 5 mg product may be appropriate for less severe symptoms.
adults 65 years and over
ask a doctor
children under 6 years of age
ask a doctor
consumers with liver or kidney disease
ask a doctor
Other information:
-
store between 20º to 25º C (68º to 77º F).
-
Phenylketonurics: Contains 1.68 mg Phenylalanine (a component of Aspartame) per 5 mg
-
Phenylketonurics: Contains 3.36 mg Phenylalanine (a component of Aspartame) per 10 mg
-
do not use if carton is opened or if blister unit is broken.
-
see bottom panel for lot number and expiration date.
-
- Inactive ingredients:
- Questions? call 1-800-313-4623
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
CADISTA NDC: 59746-285-33
Children's
Cetirizine Hydrochloride Chewable Tablets 5 mg
Antihistamine
Phenylketonurics: contains 1.68 mg Phenyalanine (a component of Aspartame ) per 5 mg tablets.
HIVES Relief
24 hour Relief of Itching due to hives
6 yrs. & older 5 mg each
TAMPER EVIDENT : Do not use if blister unit is broken or torn.
30 Chewable Tablets Orange Chewables
5 mg each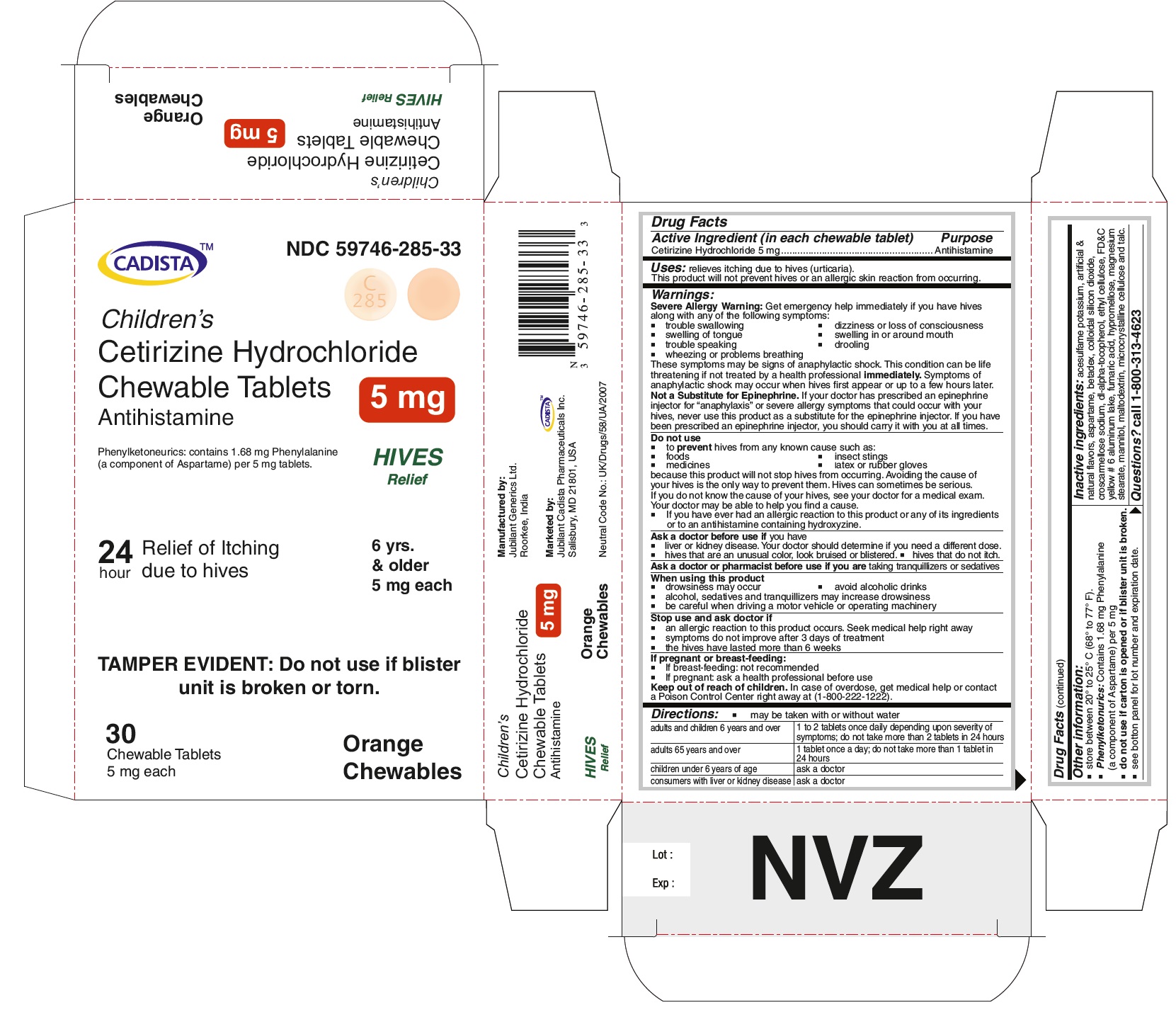
Cetirizine Hydrochloride Chewable Tablets 5 mg Hives Relief
CADISTA NDC: 59746-286-33
Children's
Cetirizine Hydrochloride Chewable Tablets 10 mg
Antihistamine
Phenylketonurics: contains 3.36 mg phenyalanine (a component of Aspartame) per 10 mg tablets.
HIVES Relief
24 hour Relief of Itching due to hives
6 yrs. & older 10 mg each
TAMPER EVIDENT : Do not use if blister unit is broken or torn.
30 Chewable Tablets Orange Chewables
10 mg each
Cetirizine Hydrochloride Chewable Tablets 10 mg Hives Relief
-
INGREDIENTS AND APPEARANCE
CETIRIZINE HYDROCHLORIDE
cetirizine hydrochloride tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 59746-285 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Cetirizine Hydrochloride (UNII: 64O047KTOA) (Cetirizine - UNII:YO7261ME24) Cetirizine Hydrochloride 5 mg Inactive Ingredients Ingredient Name Strength Acesulfame Potassium (UNII: 23OV73Q5G9) Aspartame (UNII: Z0H242BBR1) Betadex (UNII: JV039JZZ3A) Silicon Dioxide (UNII: ETJ7Z6XBU4) Croscarmellose Sodium (UNII: M28OL1HH48) .alpha.-tocopherol, Dl- (UNII: 7QWA1RIO01) Ethylcelluloses (UNII: 7Z8S9VYZ4B) Fd&c Yellow No. 6 (UNII: H77VEI93A8) Fumaric Acid (UNII: 88XHZ13131) Hypromelloses (UNII: 3NXW29V3WO) Magnesium Stearate (UNII: 70097M6I30) Mannitol (UNII: 3OWL53L36A) Maltodextrin (UNII: 7CVR7L4A2D) Cellulose, Microcrystalline (UNII: OP1R32D61U) Talc (UNII: 7SEV7J4R1U) Product Characteristics Color ORANGE Score no score Shape ROUND Size 8mm Flavor ORANGE Imprint Code C285 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59746-285-33 3 in 1 CARTON 02/19/2015 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA091116 02/19/2015 CETIRIZINE HYDROCHLORIDE
cetirizine hydrochloride tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 59746-286 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Cetirizine Hydrochloride (UNII: 64O047KTOA) (Cetirizine - UNII:YO7261ME24) Cetirizine Hydrochloride 10 mg Inactive Ingredients Ingredient Name Strength Acesulfame Potassium (UNII: 23OV73Q5G9) Aspartame (UNII: Z0H242BBR1) Betadex (UNII: JV039JZZ3A) Silicon Dioxide (UNII: ETJ7Z6XBU4) Croscarmellose Sodium (UNII: M28OL1HH48) .alpha.-tocopherol, Dl- (UNII: 7QWA1RIO01) Ethylcelluloses (UNII: 7Z8S9VYZ4B) Fd&c Yellow No. 6 (UNII: H77VEI93A8) Fumaric Acid (UNII: 88XHZ13131) Hypromelloses (UNII: 3NXW29V3WO) Magnesium Stearate (UNII: 70097M6I30) Mannitol (UNII: 3OWL53L36A) Maltodextrin (UNII: 7CVR7L4A2D) Cellulose, Microcrystalline (UNII: OP1R32D61U) Talc (UNII: 7SEV7J4R1U) Product Characteristics Color ORANGE Score no score Shape ROUND Size 11mm Flavor ORANGE Imprint Code C286 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59746-286-33 3 in 1 CARTON 12/19/2015 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA091116 02/19/2015 Labeler - Jubilant Cadista Pharmaceuticals Inc. (022490515) Registrant - Jubilant Generics Limited (650801538) Establishment Name Address ID/FEI Business Operations Jubilant Generics Limited, Roorkee 650369221 MANUFACTURE(59746-285, 59746-286)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.