STERILE WATER- water injection
Sterile Water by
Drug Labeling and Warnings
Sterile Water by is a Prescription medication manufactured, distributed, or labeled by Fresenius Kabi USA, LLC, Fresenius Kabi Deutschland GmbH. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Sterile Water for Irrigation, USP is a sterile, hypotonic, nonpyrogenic irrigating fluid or pharmaceutic aid (solvent) entirely composed of Sterile Water for Injection, USP. It is prepared by distillation and contains no antimicrobial or bacteriostatic agents or added buffers.
The pH is 5.7 (5.0-7.0)
The flexible plastic container is made from a multilayered film specifically developed for parenteral drugs. It contains no plasticizers. The solution contact layer is a copolymer of ethylene and propylene. The container is nontoxic and biologically inert. The container-solution unit is a closed system and is not dependent upon entry of external air during administration.
Not made with natural rubber latex, PVC or DEHP.
-
CLINICAL PHARMACOLOGY
Sterile Water for Irrigation is utilized for a variety of clinical indications. Because of its low refractive index (1.3325), water provides excellent visibility during endoscopic urological procedures. It is also utilized as a pharmaceutic aid, as well as in the preparation of enteral nutrient products.
Water is hypotonic and will cause hemolysis and will be readily absorbed by the tissues during surgical procedures; therefore, its use under such conditions is not recommended.
-
INDICATIONS AND USAGE
Sterile Water for Irrigation is indicated for use as an irrigating fluid or pharmaceutic aid. Sterile Water may also be used as an adjunct in the preparation of non-intravenously administered nutrient mixtures (see DOSAGE AND ADMINISTRATION).
- CONTRAINDICATIONS
-
WARNINGS
Sterile Water for Irrigation is hypotonic and will cause hemolysis, and is not recommended for use during surgical procedures.
After opening container, its contents should be used promptly to minimize the possibility of bacterial growth or pyrogen formation.
Discard unused portion of irrigating solution since it contains no preservative.
- PRECAUTIONS
- ADVERSE REACTIONS
- OVERDOSAGE
-
DOSAGE AND ADMINISTRATION
Irrigation
Use as directed by physician.
This drug product should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Nutrient Mixtures
Sterile Water for Irrigation, USP may be used to prepare non-intravenously administered nutrient mixtures. It contains no electrolytes or other added substances. Refer to preparation instructions of particular mixture to be used. The plastic container may be used for administration of non-intravenous nutrient mixture to the patient as appropriate.
-
HOW SUPPLIED
Sterile Water for Irrigation, USP is supplied sterile and nonpyrogenic in single dose flexible plastic containers.
Product Code
Unit of Sale
Each
495010
NDC: 65219-495-10
Package of 6 bagsNDC: 65219-495-01
1000 mL fill in a 1500 mL bag497020
NDC: 65219-497-20
Package of 5 bagsNDC: 65219-497-02
2000 mL fill in a 2000 mL bag499030
NDC: 65219-499-30
Package of 4 bagsNDC: 65219-499-03
3000 mL fill in a 3000 mL bagExposure of pharmaceutical products to heat should be minimized. Avoid excessive heat. Protect from freezing. Store at 20° to 25°C (68° to 77°F). [see USP Controlled Room Temperature]; however, brief exposure up to 40°C (104°F) does not adversely affect the product.
-
INSTRUCTIONS FOR USE:
Not for Injection. Not for use with pressurized irrigation systems.
Check solution container composition, lot number, and expiry date.
Irrigation solutions should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Do not use if the solution is cloudy or precipitate is present.
Check the solution container for leaks by squeezing firmly. If leaks are found, discard.
The intact port cap provides visual tamper evidence. Do not use if port cap is prematurely removed. Maintain strict aseptic technique during handling.
To Add Medication:
- 1. Identify WHITE Additive Port with arrow pointing toward solution container.
- 2. Immediately before injecting additives, break off WHITE Additive Port Cap with the arrow pointing toward solution container.
- 3. Hold base of WHITE Additive Port.
- 4. Insert needle (18 – 23 gauge) through the center of the WHITE Additive Port's resealable septum and inject additives.
- 5. Mix solution container contents thoroughly.
- 6. WHITE Additive port must be swabbed with disinfection agent before re-puncturing.
- 7. Check admixture visually for particulate matter.
Preparation for Administration
- 1. Immediately before inserting the irrigation set, break off BLUE Administration Port Cap with the arrow pointing away from the solution container.
- 2. Use non-vented irrigation set or close the air-inlet on a vented set. Refer to directions for use accompanying the irrigation set.
- 3. Hold the base of the BLUE Administration Port, twist and push spike until fully inserted.
- 4. The BLUE Administration Port contains a self-sealing septum that helps prevent leakage after removing the spike. The Administration Port is not intended to be spiked more than once.
- 5. Suspend solution container from hanger hole.
- 6. For Single Use Only. Discard unused portion.
Manufactured for:
Lake Zurich, IL 60047
Made in Germany
www.fresenius-kabi.com/us451819
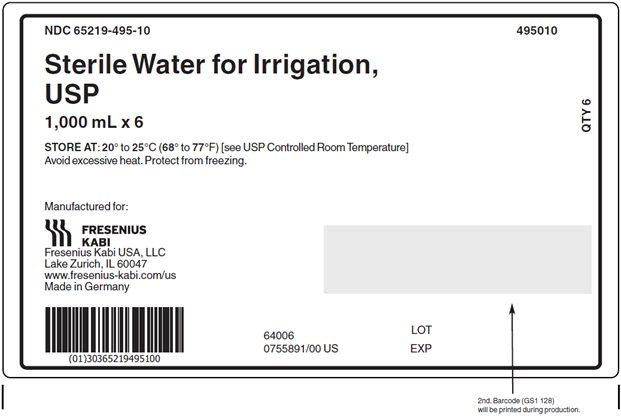
Issued: September 2024 - PACKAGE LABEL - PRINCIPAL DISPLAY – Sterile Water for Irrigation, USP 1000 mL Bag Label
-
PACKAGE LABEL - PRINCIPAL DISPLAY - Sterile Water for Irrigation, USP 1000 mL Case Label
NDC: 65219-495-10 495010
Sterile Water for Irrigation,
USP
1,000 mL x 6STORE AT: 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]
Avoid excessive heat. Protect from freezing. - PACKAGE LABEL - PRINCIPAL DISPLAY – Sterile Water for Irrigation, USP 2000 mL Bag Label
-
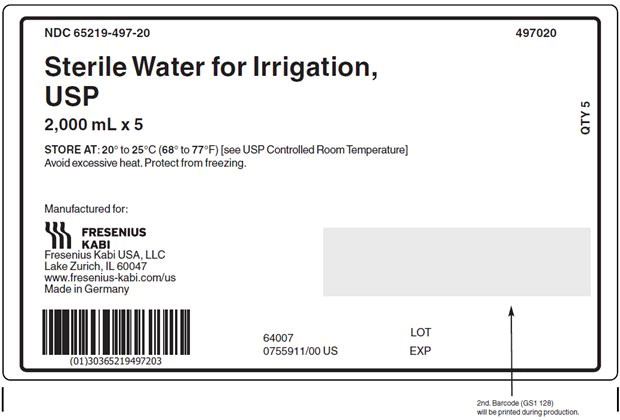
PACKAGE LABEL - PRINCIPAL DISPLAY - Sterile Water for Irrigation, USP 2000 mL Case Label
NDC: 65219-497-20 497020
Sterile Water for Irrigation,
USP
2,000 mL x 5STORE AT: 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]
Avoid excessive heat. Protect from freezing. - PACKAGE LABEL - PRINCIPAL DISPLAY – Sterile Water for Irrigation, USP 3000 mL Bag Label
-
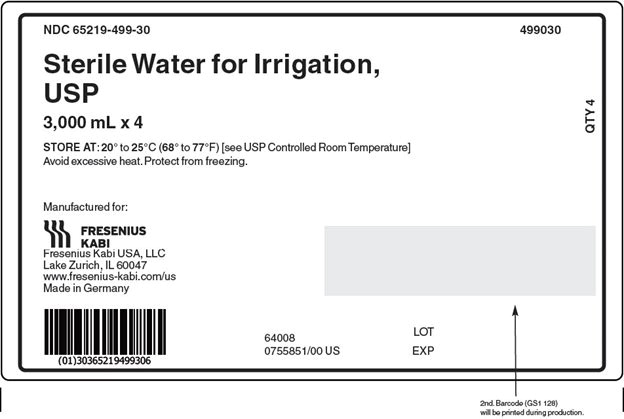
PACKAGE LABEL - PRINCIPAL DISPLAY - Sterile Water for Irrigation, USP 3000 mL Case Label
NDC: 65219-499-30 499030
Sterile Water for Irrigation,
USP
3,000 mL x 4STORE AT: 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]
Avoid excessive heat. Protect from freezing. -
INGREDIENTS AND APPEARANCE
STERILE WATER
water injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 65219-495 Route of Administration IRRIGATION Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 1 mL in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65219-495-10 6 in 1 CASE 12/19/2025 1 NDC: 65219-495-01 1000 mL in 1 BAG; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA216123 12/19/2025 STERILE WATER
water injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 65219-497 Route of Administration IRRIGATION Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 1 mL in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65219-497-20 5 in 1 CASE 12/19/2025 1 NDC: 65219-497-02 2000 mL in 1 BAG; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA216123 12/19/2025 STERILE WATER
water injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 65219-499 Route of Administration IRRIGATION Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 1 mL in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65219-499-30 4 in 1 CASE 03/03/2025 1 NDC: 65219-499-03 3000 mL in 1 BAG; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA216123 03/03/2025 Labeler - Fresenius Kabi USA, LLC (013547657) Establishment Name Address ID/FEI Business Operations Fresenius Kabi Deutschland GmbH 506719546 MANUFACTURE(65219-495, 65219-497, 65219-499) , ANALYSIS(65219-495, 65219-497, 65219-499) , API MANUFACTURE(65219-495, 65219-497, 65219-499)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.