GUAIFENESIN by Sam's West Inc / Aurohealth LLC / APL HEALTHCARE LIMITED Drug Facts
GUAIFENESIN by
Drug Labeling and Warnings
GUAIFENESIN by is a Otc medication manufactured, distributed, or labeled by Sam's West Inc, Aurohealth LLC, APL HEALTHCARE LIMITED. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
GUAIFENESIN - guaifenesin tablet, extended release
Sam's West Inc
----------
Drug Facts
Uses
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
Ask a doctor before use if you have
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- cough accompanied by too much phlegm (mucus)
Stop use and ask a doctor if
- cough lasts more than 7 days, comes back, or occurs with fever, rash, or persistent headache. These could be signs of a serious illness.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away (1-800-222-1222).
Directions
- do not crush, chew, or break tablet
- take with a full glass of water
- this product can be administered without regard for the timing of meals
- adults and children 12 years of age and over: 1 tablet every 12 hours. Do not exceed 2 tablets in 24 hours.
- children under 12 years of age: do not use
Inactive ingredients
colloidal silicon dioxide, hypromellose, magnesium stearate, microcrystalline cellulose, povidone, and pregelatinised starch (maize)
Questions?
call toll-free Monday to Friday 8:30 am to 5:00 pm EST at 1-800-406-7984.
DISTRIBUTED BY:
SAM’S WEST, INC.
BENTONVILLE, AR 72716
PRODUCT OF INDIA
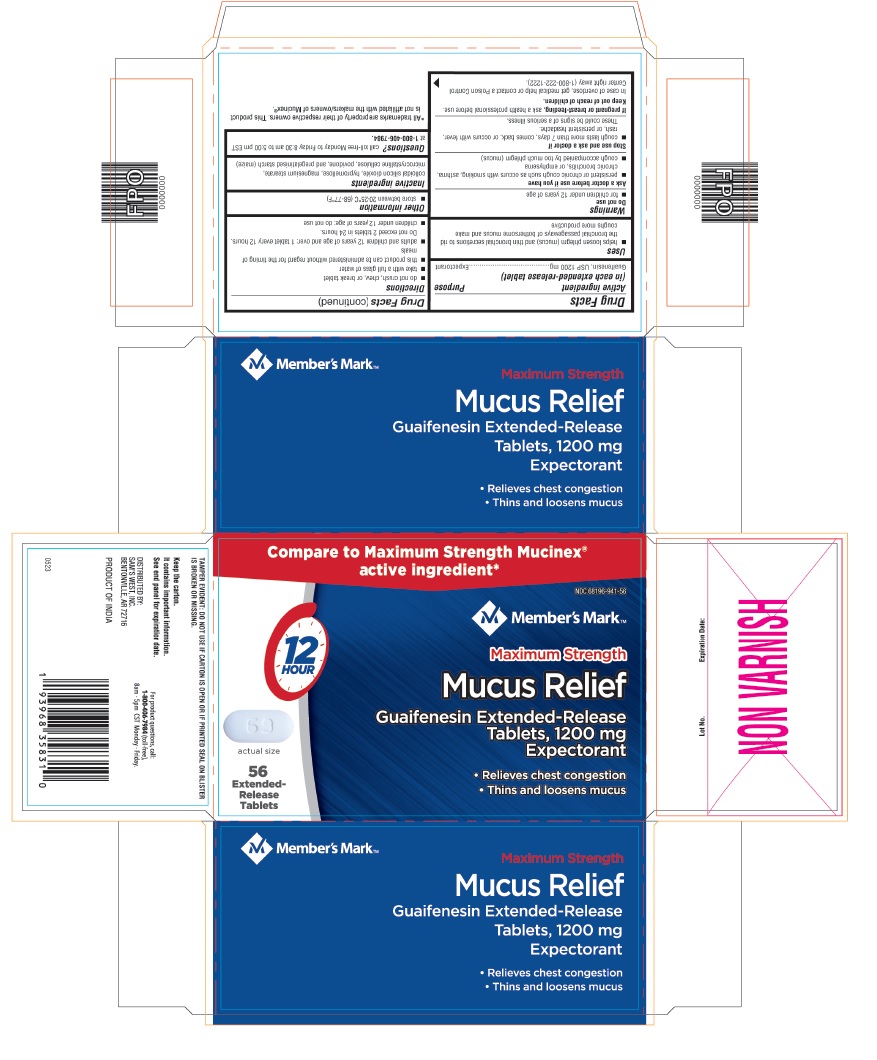
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 1200 mg (56 Extended-Release Tablets Blister Carton Label)
Compare to Maximum Strength Mucinex®
active ingredient*
NDC: 68196-941-56
Member's Mark™
Maximum Strength
Mucus Relief
Guaifenesin Extended-Release
Tablets, 1200 mg
Expectorant
- Relieves chest congestion
- Thins and loosens mucus
12
HOUR
69
actual size
56
Extended-
Release
Tablets
| GUAIFENESIN
guaifenesin tablet, extended release |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Sam's West Inc (051957769) |
| Registrant - Aurohealth LLC (078728447) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| APL HEALTHCARE LIMITED | 650918514 | ANALYSIS(68196-941) , MANUFACTURE(68196-941) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.