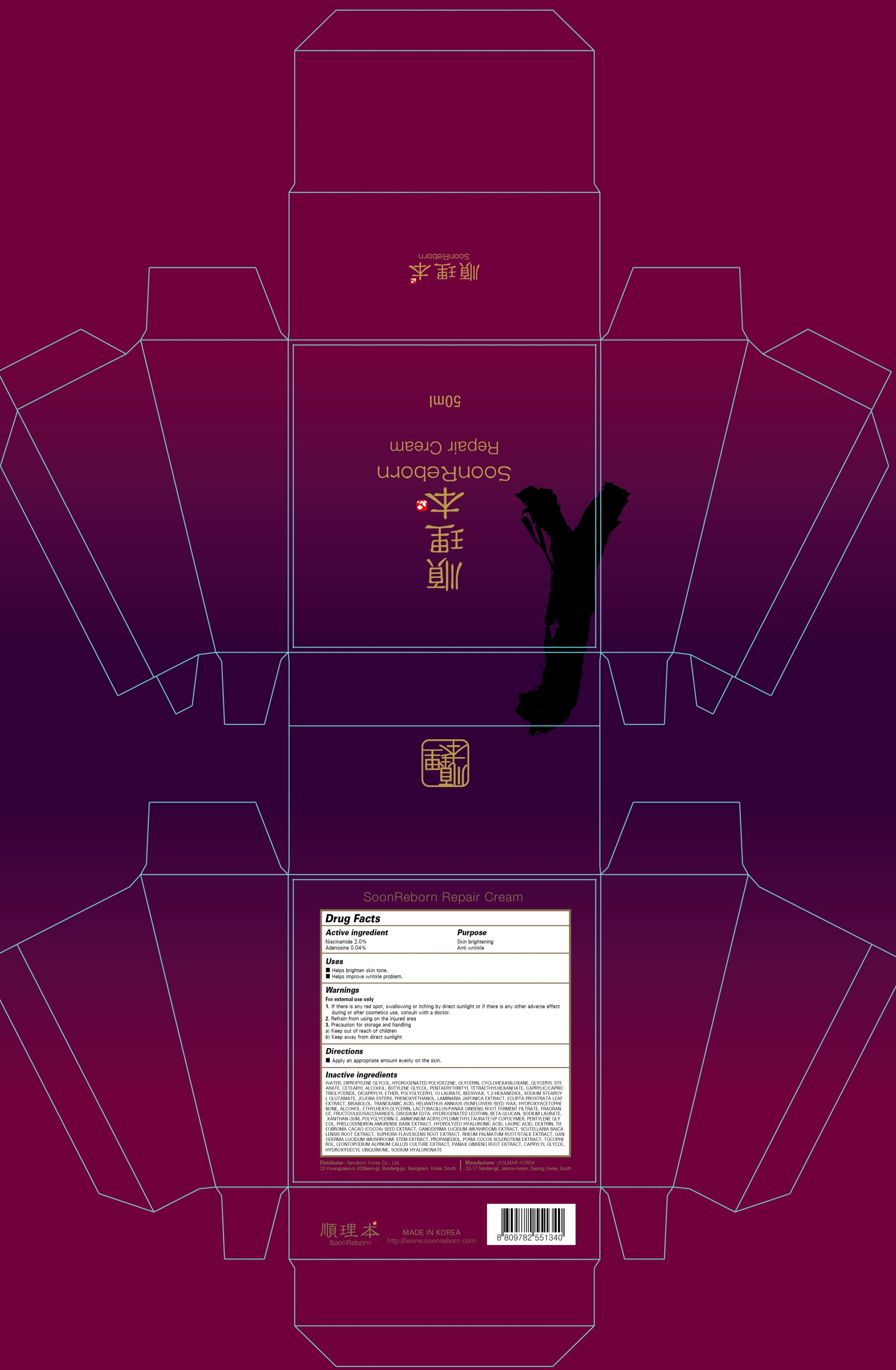
ACTIVE INGREDIENTS
Niacinamide 2.0%
Adenosine 0.04%
INACTIVE INGREDIENTS
WATER, DIPROPYLENE GLYCOL, HYDROGENATED POLYDECENE, GLYCERIN, CYCLOHEXASILOXANE, GLYCERYL STEARATE, CETEARYL ALCOHOL, BUTYLENE GLYCOL, PENTAERYTHRITYL TETRAETHYLHEXANOATE, CAPRYLIC/CAPRIC TRIGLYCERIDE, DICAPRYLYL ETHER, POLYGLYCERYL-10 LAURATE, BEESWAX, 1,2-HEXANEDIOL, SODIUM STEAROYL GLUTAMATE, JOJOBA ESTERS, PHENOXYETHANOL, LAMINARIA JAPONICA EXTRACT, ECLIPTA PROSTRATA LEAF EXTRACT, BISABOLOL, TRANEXAMIC ACID, HELIANTHUS ANNUUS (SUNFLOWER) SEED WAX, HYDROXYACETOPHENONE, ALCOHOL, ETHYLHEXYLGLYCERIN, LACTOBACILLUS/PANAX GINSENG ROOT FERMENT FILTRATE, FRAGRANCE, FRUCTOOLIGOSACCHARIDES, DISODIUM EDTA, HYDROGENATED LECITHIN, BETA-GLUCAN, SODIUM LAURATE, XANTHAN GUM, POLYGLYCERIN-3, AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER, PENTYLENE GLYCOL, PHELLODENDRON AMURENSE BARK EXTRACT, HYDROLYZED HYALURONIC ACID, LAURIC ACID, DEXTRIN, THEOBROMA CACAO (COCOA) SEED EXTRACT, GANODERMA LUCIDUM (MUSHROOM) EXTRACT, SCUTELLARIA BAICALENSIS ROOT EXTRACT, SOPHORA FLAVESCENS ROOT EXTRACT, RHEUM PALMATUM ROOT/STALK EXTRACT, GANODERMA LUCIDUM (MUSHROOM) STEM EXTRACT, PROPANEDIOL, PORIA COCOS SCLEROTIUM EXTRACT, TOCOPHEROL, LEONTOPODIUM ALPINUM CALLUS CULTURE EXTRACT, PANAX GINSENG ROOT EXTRACT, CAPRYLYL GLYCOL, HYDROXYDECYL UBIQUINONE, SODIUM HYALURONATE
PURPOSE
Skin brightening
Anti wrinkle
WARNINGS
For external use only
1. If there is any red spot, swallowing or itching by direct sunlight or if there is any other adverse effect during or after cosmetics use, consult with a doctor.
2. Refrain from using on the injured area
3. Precaution for storage and handling
a) Keep out of reach of children
b) Keep away from direct sunlight
KEEP OUT OF REACH OF CHILDREN
KEEP OUT OF REACH OF CHILDREN
Uses
■ Helps brighten skin tone.
■ Helps improve wrinkle problem.
Directions
■ Apply an appropriate amount evenly on the skin.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL