ALLERGY RELIEF- cetirizine hydrochloride solution
Allergy Relief by
Drug Labeling and Warnings
Allergy Relief by is a Otc medication manufactured, distributed, or labeled by H E B. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each 5 mL)
- Purpose
- Uses
-
Warnings
Do not use if you have ever had an allergic reaction to this product or any of its ingredients or to an antihistamine containing hydroxyzine.
Ask a doctor before use if you have liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product
- drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
Stop use and ask a doctor if an allergic reaction to this product occurs. Seek medical help right away.
-
Directions
- use only with enclosed dosing cup
- find right dose on chart below
- mL = milliliter
adults and children 6 years and over 5 mL or 10 mL once daily depending upon severity of symptoms; do not take more than 10 mL in 24 hours. adults 65 years and over 5 mL once daily; do not take more than 5 mL in 24 hours. children 2 to under 6 years of age 2.5 mL once daily. If needed, dose can be increased to a maximum of 5 mL once daily or 2.5 mL every 12 hours. Do not give more than 5 mL in 24 hours. children under 2 years of age ask a doctor consumers with liver or kidney disease ask a doctor - Other information
- Inactive ingredients
- Questions?
-
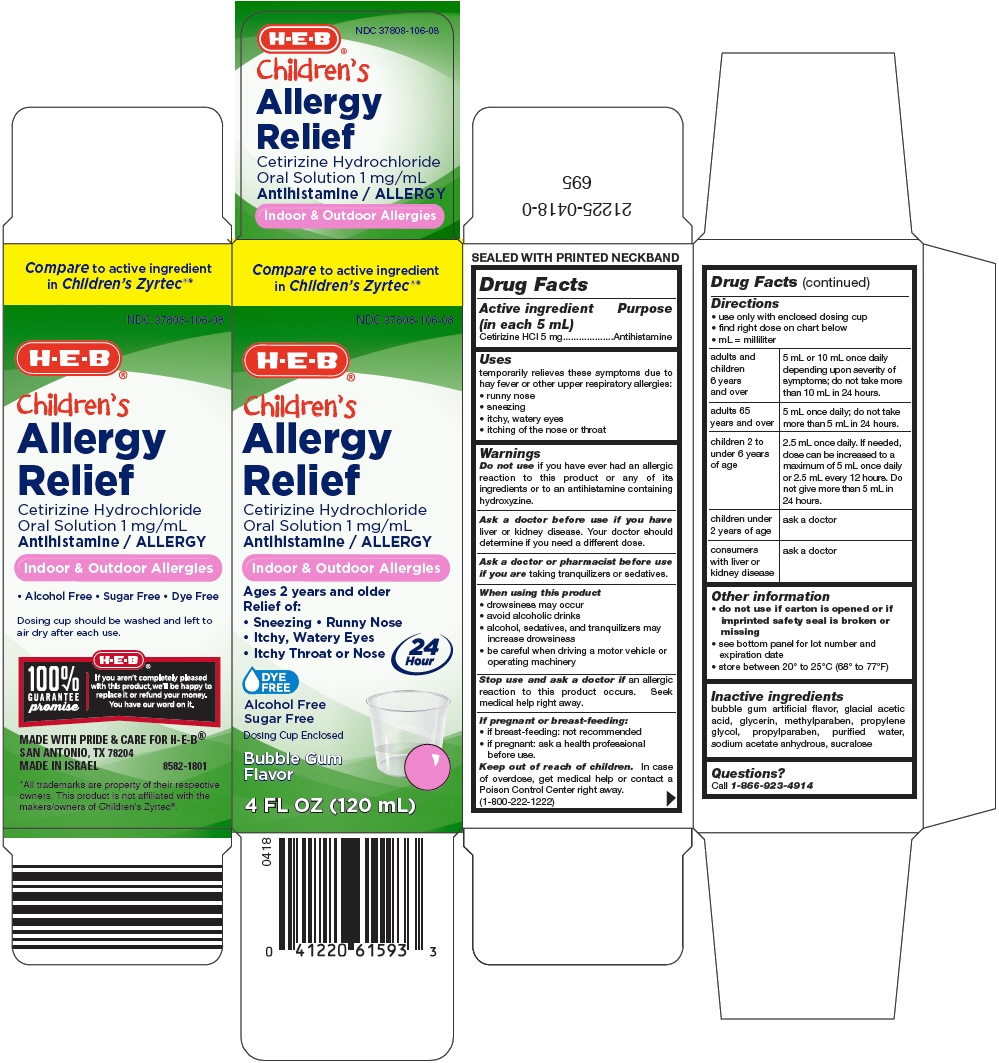
PRINCIPAL DISPLAY PANEL - 120 mL Bottle Carton
Compare to active ingredient
in Children's Zyrtec®*NDC: 37808-106-08
H-E-B®
Children's
Allergy
ReliefCetirizine Hydrochloride
Oral Solution 1 mg/mL
Antihistamine / ALLERGYIndoor & Outdoor Allergies
Ages 2 years and older
Relief of:- Sneezing Runny Nose
- Itchy, Watery Eyes
- Itchy Throat or Nose
24
HourDYE
FREEAlcohol Free
Sugar FreeDosing Cup Enclosed
Bubble Gum
Flavor4 FL OZ (120 mL)
-
INGREDIENTS AND APPEARANCE
ALLERGY RELIEF
cetirizine hydrochloride solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 37808-106 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Cetirizine Hydrochloride (UNII: 64O047KTOA) (Cetirizine - UNII:YO7261ME24) Cetirizine Hydrochloride 1 mg in 1 mL Inactive Ingredients Ingredient Name Strength acetic acid (UNII: Q40Q9N063P) glycerin (UNII: PDC6A3C0OX) methylparaben (UNII: A2I8C7HI9T) propylene glycol (UNII: 6DC9Q167V3) propylparaben (UNII: Z8IX2SC1OH) water (UNII: 059QF0KO0R) sodium acetate anhydrous (UNII: NVG71ZZ7P0) sucralose (UNII: 96K6UQ3ZD4) Product Characteristics Color YELLOW (colorless to slightly yellow) Score Shape Size Flavor BUBBLE GUM (Sugar Free) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 37808-106-08 1 in 1 CARTON 04/12/2018 1 120 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA201546 05/20/2011 Labeler - H E B (007924756)
Trademark Results [Allergy Relief]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ALLERGY RELIEF 98236984 not registered Live/Pending |
Dmytro Kononenko 2023-10-24 |
 ALLERGY RELIEF 90457167 not registered Live/Pending |
American Textile Company, Inc. 2021-01-10 |
 ALLERGY RELIEF 78838437 3358249 Live/Registered |
Meshbesher Health Corporation 2006-03-16 |
 ALLERGY RELIEF 76619855 3066888 Live/Registered |
AMERICAN TEXTILE COMPANY 2004-11-09 |
 ALLERGY RELIEF 74668018 not registered Dead/Abandoned |
NaturaLife Corporation 1995-05-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.