VYJUVEK by Krystal Biotech, Inc. VYJUVEK kit
VYJUVEK by
Drug Labeling and Warnings
VYJUVEK by is a Prescription medication manufactured, distributed, or labeled by Krystal Biotech, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use VYJUVEK safely and effectively. See full prescribing information for
VYJUVEK.
VYJUVEK® (beremagene geperpavec-svdt) biological suspension mixedwith excipient gel for topical applicationInitial U.S. Approval: 2023RECENT MAJOR CHANGES
INDICATIONS AND USAGE
VYJUVEK is a herpes-simplex virus type 1 (HSV-1) vector-based gene therapy indicated for the treatment of wounds in adult and pediatric patients with dystrophic epidermolysis bullosa (DEB) with mutation(s) in the collagen type VII alpha 1 chain (COL7A1)gene. (1)
DOSAGE AND ADMINISTRATION
For topical application only.
Age Range Maximum Weekly Dose (PFU) Maximum Weekly Volume (mL)* <3 years old 2×10 9 1 ≥3 years old 4×10 9 2 PFU=plaque forming units; mL=milliliter
*Maximum weekly volume after mixing VYJUVEK biological suspension with excipient gel
Apply VYJUVEK gel to the selected wound(s) in droplets spaced evenly within the wound, approximately 1cm-by-1cm apart. (2.3)
DOSAGE FORMS AND STRENGTHS
VYJUVEK is a biological suspension, mixed into excipient gel, for topical application. VYJUVEK biological suspension is supplied as a 1 mL extractable volume in a single dose vial at a nominal concentration of 5×10 9PFU/mL. The excipient gel is supplied as a 1.5 mL fill volume in a separate single use vial. VYJUVEK biological suspension (1 mL) is mixed into the excipient gel vial prior to administration as VYJUVEK gel. ( 3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Accidental Exposure to VYJUVEK: Avoid direct contact with treated wounds and dressings of treated wounds until the next dressing change, following application. Clean the affected area if accidental exposure occurs. (5.1)
ADVERSE REACTIONS
The most common adverse drug reactions (incidence >5%) were itching, chills, redness, rash, cough, and runny nose. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Krystal Biotech, Inc. at 1-844-557-9782 or FDA at 1-800-FDA-1088 or http://www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 9/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dose
2.2 Preparation
2.3 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Accidental Exposure to VYJUVEK
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/ STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Dose
For topical application on wounds only.
- The recommended dose of VYJUVEK gel is based on age (Table 1). VYJUVEK gel is applied topically to wound(s) once a week.
Table 1 Maximum Weekly Dose of VYJUVEK by Age Age Range Maximum Weekly Dose (PFU) Maximum Weekly Volume (mL)* <3 years old 2×10 9 1 ≥3 years old 4×10 9 2 PFU=plaque forming unit; mL=milliliter
*Maximum weekly volume is the volume after mixing VYJUVEK biological suspension with excipient gel.
- It may not be possible to apply VYJUVEK gel to all the wounds at each treatment visit.
- Apply VYJUVEK gel to wounds until they are closed before selecting new wound(s) to treat.
- Prioritize weekly treatment to previously treated wounds if they re-open. [ see Administration (2.3)]
- If a dose is missed, apply VYJUVEK gel as soon as possible and resume weekly dosing thereafter.
2.2 Preparation
Important Preparation Instructions
- Prepare VYJUVEK gel at the pharmacy by mixing the VYJUVEK biological suspension into the excipient gel for immediate use. [see Storage and Handling (16.2)]
- The VYJUVEK gel prepared at the pharmacy, should be applied by a healthcare professional (HCP), patient, or caregiver either at a healthcare professional setting (e.g., clinic) or at a home setting.
- Individuals who are pregnant should not prepare or apply VYJUVEK gel and should avoid direct contact with the treated wounds or dressings from treated wounds [see Accidental Exposure to VYJUVEK( 5.1)].
Below is the list of supplies needed for VYJUVEK gel preparation:
- One (1) carton containing one (1) VYJUVEK biological suspension vial and one (1) excipient gel vial (Figure 1)
- Two (2) 18-gauge needles
- Two (2) to four (4) 1 mL administration syringes
- One (1) 3 mL preparation syringe
- Two (2) to four (4) syringe caps
- Protective gloves
- 70% isopropyl alcohol pads
- Biohazard container
- Labels for administration syringes
- Virucidal agent for clean-up
Follow the steps below for VYJUVEK gel preparation.
PREPARE THE PREPARATION SYRINGE
1. Wash hands and put on protective gloves.
2. Remove both vials from the carton and thaw the VYJUVEK biological suspension vial and the excipient gel vial at room temperature for AT LEAST 20 minutes (Figure 1).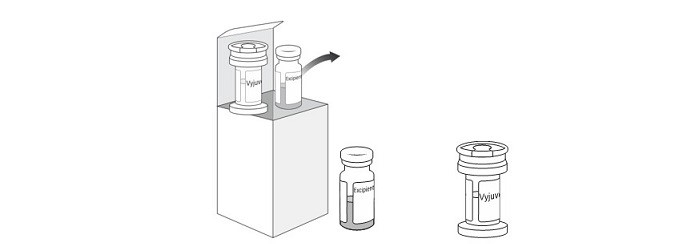
Figure 1 Carton containing the VYJUVEK biological suspension vial and excipient gel vial
Note: Visually inspect the vials to ensure both are in liquid form and completely thawed. Excipient gel is more viscous and will take longer to thaw (Figure 2).
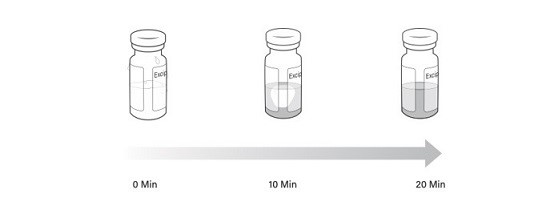
Figure 2 Timelapse of excipient gel thaw from 0 minutes to 20 minutes
Note: Once either the VYJUVEK biological suspension or the excipient gel is thawed, do not refreeze.
3. Invert the VYJUVEK biological suspension vial 4-5 times. Do not invert the excipient gel vial.
4. Remove the caps from the vials and clean each vial stopper with a 70% isopropyl alcohol pad. Allow them to dry.
5. Aseptically connect an 18-gauge needle to the 3 mL preparation syringe.
6. Remove the needle cap and puncture the VYJUVEK biological suspension vial stopper.
7. Hold the vial at 45 to 90 degrees and withdraw 1 mL of VYJUVEK biological suspension into the preparation syringe (Figure 3).
8. Remove the preparation syringe (still connected to the needle) containing 1 mL of VYJUVEK biological suspension from the vial. Do NOT engage the safety lock.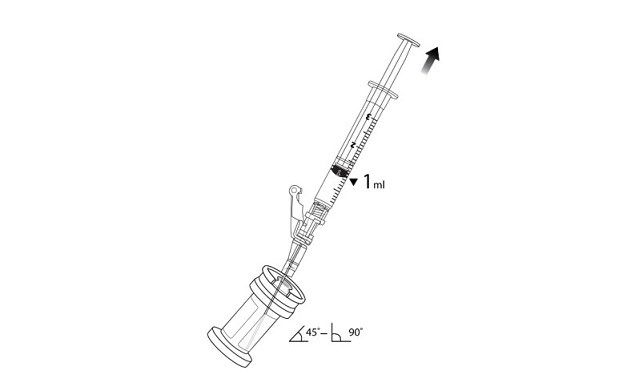
Figure 3 The removal of 1 mL of biological suspension using the preparation syringe
9. Discard the VYJUVEK biological suspension vial in the biohazard waste.
10. Puncture the clean excipient gel stopper and transfer the VYJUVEK biological suspension into the excipient gel vial (Figure 4).
Figure 4 Transferring VYJUVEK biological suspension to excipient gel vial
11. WITHOUT REMOVING THE NEEDLE from the excipient gel vial, lift the tip of the needle above the liquid (Figure 5)and pull the plunger back to the 1 mL mark (Figure 6) to vent the gel vial following the addition of the 1 mL of suspension.
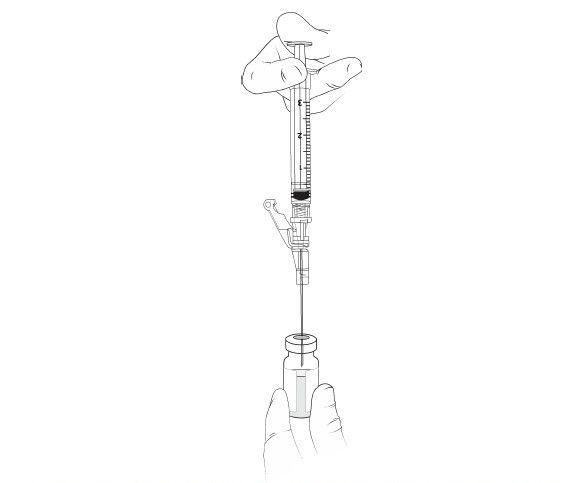
Figure 5 The needle above the liquid without removal of any material
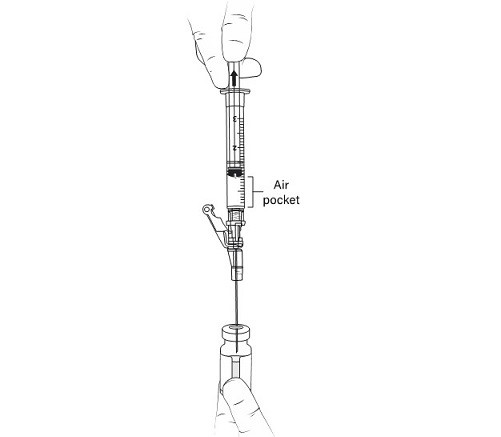
Figure 6 Pulling the plunger back to the 1 mL mark to remove air pocket
12. Remove the preparation syringe with the 1 mL of air and engage the safety lock.
13. Discard the preparation syringe and needle into the biohazard waste.
14. Place a 70% isopropyl alcohol pad on top of the excipient gel stopper and hold it tightly in place.
15.Shake VIGOROUSLY for 10 SECONDS (Figure 7).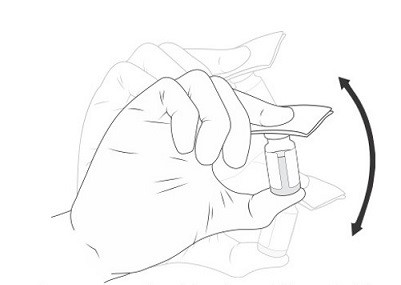
Figure 7 Manually mixing the excipient gel vial
Note: The mixture of VYJUVEK biological suspension and excipient gel is referred to as VYJUVEK gel.
PREPARE THE ADMINISTRATION SYRINGES
16. Aseptically connect an 18-gauge needle to the first 1 mL administration syringe and remove the needle cap.
17. Insert the 18-gauge needle into the excipient vial containing VYJUVEK gel (Figure 8).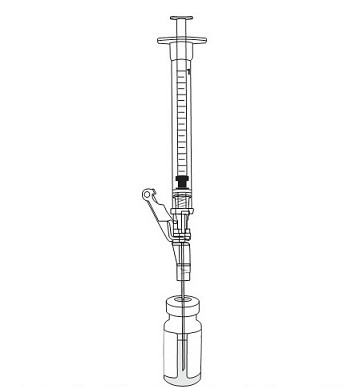
Figure 8 Needle within VYJUVEK gel prior to withdraw
18. Tilt the vial 45 to 90 degrees and withdraw 0.5 mL of VYJUVEK gel (Figure 9).
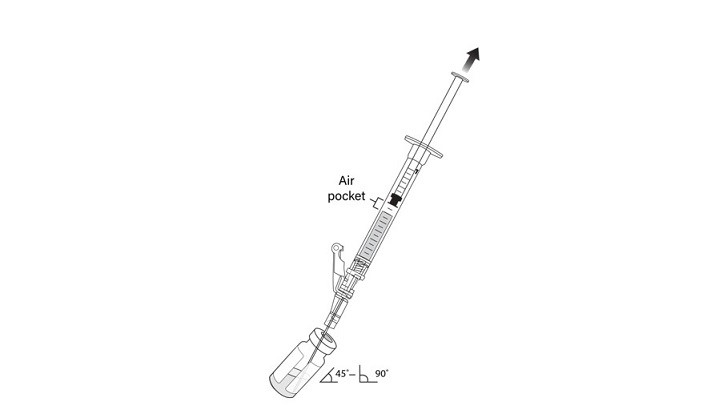
Figure 9 Administration syringe withdrawing 0.5 mL of VYJUVEK gel with air pocket visible
Note: An air pocket may form near the plunger when extracting VYJUVEK gel.
19. DO NOT REMOVE THE NEEDLE FROM THE VIAL; lift the tip of the needle above the VYJUVEK gel and disconnect the administration syringe containing 0.5 mL of mixed VYJUVEK (Figure 10).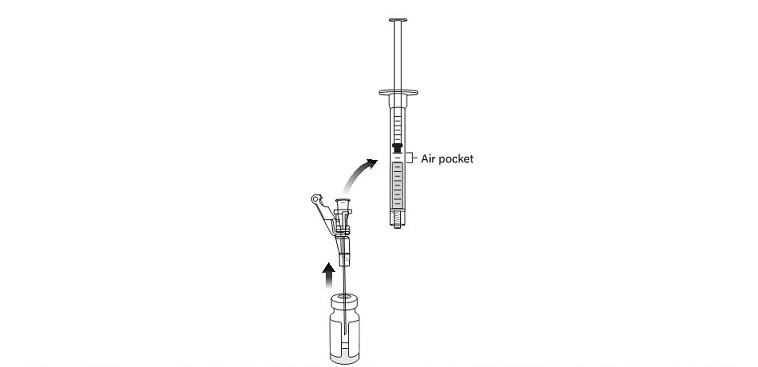
Figure 10 Disconnecting the administration syringe containing VYJUVEK gel with air pocket visible
Note: Leave the needle in the excipient gel vial stopper.
20. DO NOT flick the syringe to remove the air pocket. Manipulate the plunger up and down, until all air pockets have been removed (Figure 11)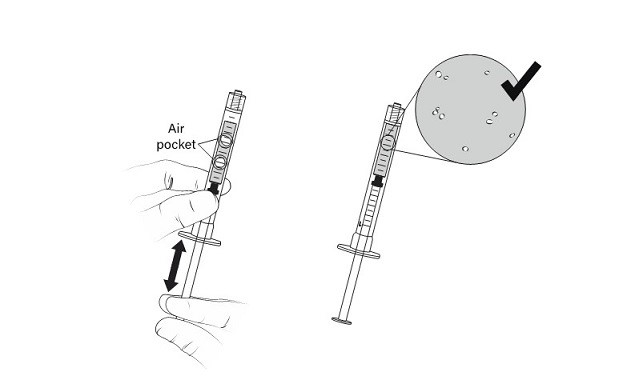
Figure 11 Manipulation of administration syringe plunger to remove air pockets
21. Cap the administration syringe and set aside (Figure 12).
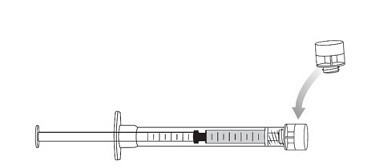
Figure 12 Capped syringe
22. Connect a new 1 mL administration syringe to the needle that remains in the excipient gel vial stopper (with the tip of the needle ABOVE the VYJUVEK gel) (Figure 13).
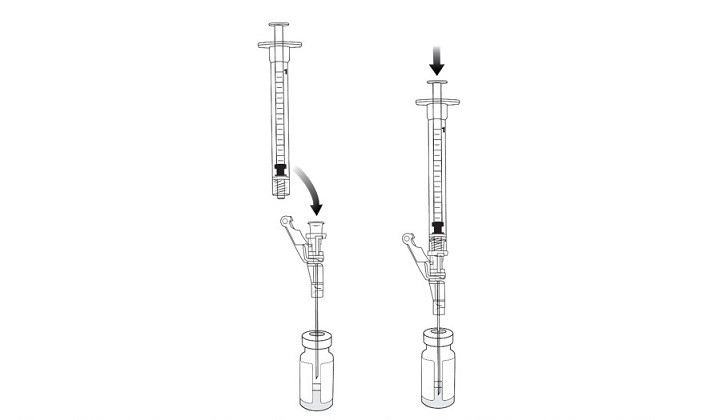
Figure 13 Connecting an administration syringe to the needle within the excipient gel vial stopper
23. Place the bevel of the needle in the VYJUVEK gel, tilt the vial 45 to 90 degrees and withdraw 0.5 mL of VYJUVEK gel into the administration syringe [see steps 17 and 18: Figure 8 and Figure 9)].
24. Complete steps 19, 20, and 21 above to disconnect the administration syringe and remove the air pockets, prior to capping the administration syringe (Figure 10, Figure 11, and Figure 12).
25. Repeat steps 22, 23, and 24 if two (2) additional administration syringes are required (each containing 0.5 mL of VYJUVEK gel).
Note: Administration syringes are labeled as syringe #1, #2, #3, and #4.
26. Discard the excipient gel vial (with the needle within the vial stopper) into the biohazard container.
27. Clean all surfaces that may have come in contact with VYJUVEK biological suspension or gel and treat all spills with a virucidal agent such as 70% isopropyl alcohol, 6% hydrogen peroxide, or <0.4% ammonium chloride. Blot using absorbent materials.
28. Dispose all materials (e.g., vial, syringe, needle, cleaning materials) that may have come in contact with VYJUVEK biological suspension or gel into a biohazard container.
29. Place the capped administration syringes containing the VYJUVEK gel in a sealable plastic bag.
30. Place the sealable plastic bag with administration syringes into an appropriate insulated or temperature regulated secondary container at 2° to 8°C (35.6° to 46.4°F) for transport from the preparation site to the administration site.2.3 Administration
Below is the list of supplies needed for VYJUVEK gel administration:
- The administration syringes
- Hydrophobic dressing
- Scissors
- Standard dressing
- Protective gloves
- Biohazard container
- Virucidal agent for clean-up
VYJUVEK GEL ADMINISTRATION
Note: The administration syringe should be primed prior to the initial application by pulling the plunger down and pushing it upwards, so that a small droplet of VYJUVEK gel forms at the tip of the syringe.
Follow the steps below for VYJUVEK gel administration.
1. Apply VYJUVEK gel to the selected wound(s) in droplets spaced evenly within the wound, approximately 1cm-by-1cm apart (width of a fingertip). The resulting droplet pattern should loosely resemble a grid. Avoid touching the administration syringe to the skin and/or wound (Figure 14).
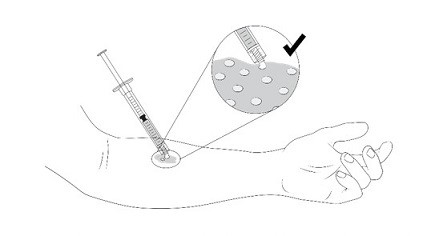
Figure 14 Application of VYJUVEK gel to the wound
Table 2below provides a reference on dose per approximate size of the wound.
Dose by Wound Size
PFU= plaque forming unit; mL= milliliter; cm= centimeter
* For wound area over 60 cm 2, recommend calculating the total dose based on Table 2 until the maximum weekly dose in Table 1 is reached.Wound Area (cm 2) Dose (PFU) Volume (mL) < 20
4×10 8
0.2
20 to < 40
8×10 8
0.4
40 to 60
1.2×10 9
0.6
2. Use the clean scissors to cut the hydrophobic dressing to a size slightly larger than the wound and place the dressing atop the VYJUVEK gel droplets (Figure 15).
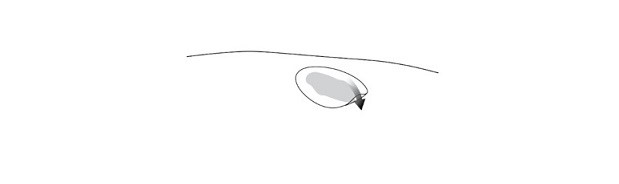
Figure 15 Placing the hydrophobic dressing over the treated wound
3. Use the scissors to cut the standard dressing used by the patient to a size slightly larger than the hydrophobic dressing and place the standard dressing atop the hydrophobic dressing (Figure 16).
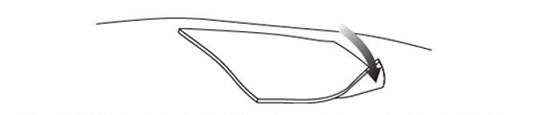
Figure 16 Placing the standard dressing over the hydrophobic dressing
4. Clean all surfaces that may have come in contact with VYJUVEK gel and treat all spills with a virucidal agent such as 70% isopropyl alcohol, 6% hydrogen peroxide or <0.4% ammonium chloride. Blot using absorbent materials.
5. Dispose all materials (e.g., syringe, cleaning materials) that may have come in contact with VYJUVEK biological suspension or gel into a biohazard container.
6. Discard unused administration syringes containing the VYJUVEK gel after preparation into a biohazard container [ see Storage and Handling (16.2)] .
7. The dressing should be left in place until the next dressing change after VYJUVEK gel application. Once the VYJUVEK dressings are removed, the patient may continue with their standard of care. -
3 DOSAGE FORMS AND STRENGTHS
VYJUVEK is an opalescent yellow to colorless biological suspension, mixed into excipient gel, for topical application. VYJUVEK biological suspension is supplied as a 1 mL extractable volume in a single-use vial with a green cap, at a nominal concentration of 5×10 9PFU/mL. The excipient gel is a clear viscous solution supplied as a 1.5 mL fill volume in a separate single-use vial with a blue cap.
VYJUVEK biological suspension (1 mL) is mixed into the excipient gel vial prior to administration.
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Accidental Exposure to VYJUVEK
Accidental exposure to VYJUVEK may occur to close contacts and caregivers. VYJUVEK is a genetically modified, herpes-simplex virus type 1 vector-based, replication-deficient, non-integrating gene therapy. VYJUVEK will not replicate in the patient’s cells and does not integrate into the patient cells’ native genetic material. For precautions,
- Avoid direct contact with treated wounds (e.g., touching or scratching) and dressings of treated wounds until the next dressing change.
- Wear protective gloves when assisting patients with changing wound dressings and handling the disposal.
- In the event of an accidental exposure through a splash to the eyes or mucous membranes, flush with clean water for at least 15 minutes.
-
6 ADVERSE REACTIONS
The most common adverse reactions (>5%) were itching, chills, redness, rash, cough, and runny nose.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety data described in this section reflects exposure to VYJUVEK gel in a randomized, intra-patient placebo‑controlled study. A total of 31 patients with dystrophic epidermolysis bullosa (DEB), including 30 patients with autosomal recessive DEB and one patient with autosomal dominant DEB received topical administration of VYJUVEK gel to their wounds. The age of the patients ranged from 1 year to 44 years (mean age 17 years). Of the 31 patients, 19 (61%) were pediatric patients (less than 17 years of age), and 11 (36%) were females. Each patient received weekly topical application of VYJUVEK gel at one or more wound sites and placebo at a matching wound site as an intra-subject comparator. The median duration of exposure to VYJUVEK gel was 25 weeks. The most frequent adverse reactions (incidence >5%) observed in the study are summarized in Table 3. There were no discontinuations due to adverse reactions.
Table 3Adverse Reactions (incidence >5%) Following Treatment with VYJUVEK gel (n =31)
Adverse Reactions
Patients n (%)
Itching
3 (10)
Chills
3 (10)
Redness
2 (6)
Rash
2 (6)
Cough
2 (6)
Runny Nose
2 (6)
In addition, the safety profile of VYJUVEK in two patients with autosomal recessive DEB (RDEB) of six and seven months of age, respectively, who received topical VYJUVEK gel weekly in an open-label study was similar to the safety profile of VYJUVEK observed in the randomized, intra-patient placebo‑controlled study described above.
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no data with VYJUVEK gel use in pregnant women to inform a drug-associated risk. Animal developmental and reproductive toxicity studies have not been conducted with VYJUVEK.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risks of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
If the patient becomes pregnant while being administered VYJUVEK gel, the patient should be apprised of the potential hazards to the fetus and neonate. Women of childbearing potential should be advised to use an effective method of contraception to prevent pregnancy during treatment with VYJUVEK gel.
8.2 Lactation
Risk Summary
There is no information available on the presence of VYJUVEK in human milk, the effects on the breastfed infant, or the effects on milk production. Animal lactation studies have not been conducted with VYJUVEK.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for VYJUVEK and any potential adverse effects on the breastfed child from VYJUVEK or from the underlying maternal condition.
8.3 Females and Males of Reproductive Potential
No nonclinical or clinical studies were performed to evaluate the effect of VYJUVEK on fertility.
8.4 Pediatric Use
The safety and effectiveness of VYJUVEK have been established in pediatric patients with dystrophic epidermolysis bullosa (DEB). The use in pediatric patients (0 to 16 years of age) was supported by evidence from adequate and well-controlled study which included 19 pediatric patients 1 year of age and older. [ see Adverse Reactions ( 6) Clinical Studies (14)].
-
11 DESCRIPTION
VYJUVEK (beremagene geperpavec-svdt) is a suspension of an HSV-1 vector-based gene therapy, mixed with the supplied sterile excipient gel for topical application on wounds. VYJUVEK is a live, replication defective HSV-1-based vector that has been genetically modified to express the human type VII collagen (COL7) protein. The parental virus for VYJUVEK was a primary isolate, which was subsequently altered using recombinant methods to result in gene deletions and insertions.
VYJUVEK is an opalescent yellow to colorless biological suspension following thaw from its frozen state. Each 1 mL VYJUVEK vial contains 5×10 9PFU/mL of VYJUVEK in a solution of 100.0 mL/L Glycerol, 8.0 mg/mL Sodium Chloride, 2.16 mg/mL Sodium Phosphate Dibasic, 0.2 mg/mL Potassium Chloride, 0.2 mg/mL Potassium Phosphate Monobasic.
The excipient gel is a clear viscous solution, following thaw from its frozen state. Each 1.5 mL Excipient Gel vial contains 44 mg/mL Hydroxypropyl Methylcellulose in a solution of 0.91 mg/mL Tromethamine, 9.0 mg/mL Sodium Chloride, 0.726 mg/mL Sodium Phosphate Dibasic, 0.21 mg/mL Potassium Phosphate Monobasic.
VYJUVEK biological suspension is mixed into excipient gel prior to administration. After mixing, VYJUVEK gel consists of 5×10 9PFU in a volume of 2.5 mL.
Neither VYJUVEK biological suspension nor the excipient gel contains preservatives.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Dystrophic epidermolysis bullosa (DEB) is caused by mutation(s) in the COL7A1gene, which results in reduced or absent levels of biologically active COL7.
Upon topical application to the wounds, VYJUVEK can transduce both keratinocytes and fibroblasts. Following entry of VYJUVEK into the cells, the vector genome is deposited in the nucleus. Once in the nucleus, transcription of the encoded human COL7A1is initiated. The resulting transcripts allow for production and secretion of COL7 by the cell in its mature form. These COL7 molecules arrange themselves into long, thin bundles that form anchoring fibrils. The anchoring fibrils hold the epidermis and dermis together and are essential for maintaining the integrity of the skin. Patients with autosomal dominant DEB (DDEB) have lower than normal functional anchoring fibrils, and patients with RDEB have no functional anchoring fibrils.
12.2 Pharmacodynamics
The pharmacodynamic activity (expression and localization of COL7 transgene) of VYJUVEK gel was demonstrated in an initial clinical study (n=6 patients). Linear deposition of the non-collagenous domain 1 (NC1) and domain 2 (NC2) of COL7 were observed at the dermal-epidermal junction in skin biopsies harvested after VYJUVEK treatment.
12.3 Pharmacokinetics
In an initial clinical study, viral vector DNA was detected in skin swab samples in all nine treated patients, with maximum level ranging from 5.1×10 4to 4.1×10 8vector genomes. In 6 out of 9 patients (67%), negative shedding was confirmed with three measurements below limit of detection within 8 weeks of treatment with VYJUVEK. No viral vector DNA was detected in blood or urine.
In the 31-patient randomized, double-blind, intra-patient placebo-controlled trial, systemic and potential environmental exposure assessments were conducted at weekly clinical site visits via quantification of VYJUVEK genomes in blood, urine, skin swabs, and bandage samples (vector shedding) using a validated qPCR assay, and detection of infectious viral particles in skin swabs (infectivity) using a validated plaque titer assay.
All blood samples and all but one urine sample collected throughout the study were below the limit of detection. Skin swabs from 19 of the 31 patients (61%) were positive for viral vector following treatment with VYJUVEK. Negative shedding from skin swabs was achieved in 16 of the 19 patients (84%) within six weeks following treatment with VYJUVEK. Most wound dressings (94%, 29/31) contained a range of detectable vector genomes. However, no extracellular infectious particles were detected on the skin surface of any patient at any timepoint tested, after topical VYJUVEK application.
12.6 Immunogenicity
There was minimum potential for systemic exposure to VYJUVEK. Antibodies against the viral vector (HSV-1) and transgene protein (COL7) were evaluated in a subset of patients in the randomized, intra-patient placebo-‑controlled clinical study. A total of 64% of evaluated patients (14/22) were anti-HSV-1 antibody positive at baseline. Six of the 8 anti-HSV-1 seronegative patients seroconverted by Week 26 following treatment with VYJUVEK. For patients with available matched baseline and end-of-study serum samples, anti‑drug antibodies (ADAs) to COL7 were detected in 72% (13/18) of patients treated with VYJUVEK for up to 26 weeks. Data are limited to perform correlative assessment on the impact of ADA on pharmacodynamic activity.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
The efficacy of VYJUVEK gel was evaluated in one randomized, double-blind, intra-patient placebo-controlled study (NCT04491604). The study enrolled patients with dystrophic epidermolysis bullosa (DEB) with genetically confirmed mutation(s) in the COL7A1 gene and who had two cutaneous wounds with similar size, appearance, and anatomical location. Two comparable wounds in each patient were selected and randomized to receive either topical application of VYJUVEK gel or the placebo (excipient gel) weekly for 26 weeks..
A total of 31 patients were treated with VYJUVEK and placebo. The demographic characteristics of the population were as follows: the mean age was 17 years (range 1 to 44 years) including 19 pediatric patients (aged 1 to 16 years), 20 patients (65%) were male, 20 patients (65%) were White, 6 patients (19%) were Asian, and 5 patients (16%) were American Indian or Alaskan Native. Thirty patients had autosomal recessive DEB
and one patient had autosomal dominant DEB. The size of the VYJUVEK gel-treated wounds ranged from 2 to 57 cm 2, with 74% of wounds < 20 cm 2and 19% from 20 to < 40 cm 2. The size of the placebo gel-treated wounds ranged from 2 to 52 cm 2, with 71% of wounds < 20 cm 2 and 26% from 20 to < 40 cm 2.Primary efficacy outcome measure was improved wound healing defined as the difference in the proportion of complete (100%) wound closure at 24 Weeks confirmed at two consecutive study visits 2 weeks apart, assessed at Weeks 22 and 24 or at Weeks 24 and 26, between the VYJUVEK gel-treated and the placebo gel-treated wounds. Other efficacy outcome measures were the difference in the proportion of complete wound closure assessed at Weeks 8 and 10 or at Weeks 10 and 12 between the VYJUVEK gel-treated and the placebo gel-treated wounds. Complete (100%) wound closure was defined as durable wound closure evaluated at two consecutive visits two weeks apart. The efficacy results are summarized in Table 4.
Table 4Summary of the efficacy results for VYJUVEK gel
Wound Closure Assessment Timepoints
Complete Wound Closure,
n (%)
VYJUVEK gel
(N=31)
Complete Wound Closure,
n (%)
Placebo gel
(N=31)
Treatment Difference
(95% CI)
p value
Weeks 22 & 24 or Weeks 24 & 26
20 (65)
8 (26)
39%
(14, 63)
0.012
Weeks 8 & 10 or Weeks 10 & 12
21 (68)
7 (23)
45%
(22, 69)
0.003
-
16 HOW SUPPLIED/ STORAGE AND HANDLING
16.1 How Supplied
Each carton of VYJUVEK (NDC: 82194-510-02) contains one single-dose vial of VYJUVEK biological suspension and one single-dose vial of excipient gel.
VYJUVEK biological suspension (inner NDC: 82194-501-01), green cap, is supplied as a 1 mL extractable volume in a single-use, single-dose vial containing 5×10 9PFU/mL.
Excipient gel (inner NDC: 82194-001-01), blue cap, is supplied as a 1.5 mL fill volume in a separate single-use, single-dose vial.
16.2 Storage and Handling
Store the VYJUVEK carton in the pharmacy at -15°C to -25°C (5°F to -13°F). If a freezer is not available, the carton can be refrigerated in the pharmacy (2° to 8°C (35.6° to 46.4°F)) for up to 1 month.
Prior to use, VYJUVEK requires mixing into excipient gel in the pharmacy.
Administration syringes containing the VYJUVEK gel may remain at room temperature (20 to 25°C (68° to 77°F)) for up to 8 hours prior to application. If immediate use is not possible, administration syringes can be kept in an appropriate insulated or temperature regulated secondary container for up to 48 hours at 2° to 8°C (35.6° to 46.4°F).
Discard material if it falls out of the parameters described above.
VYJUVEK is a replication deficient HSV-1-based gene therapy. See Dosage and Administration(2)for appropriate handling, preparation, application, and disposal of materials. Follow universal biohazard precautions for handling.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient or caregiverto read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Advise patients and caregivers of the following precautions prior to and during treatment with VYJUVEK gel:
- Avoid direct contact with treated wounds (e.g., touching and scratching) and dressings of treated wounds until the next dressing change following VYJUVEK gel application. In the event of accidental exposure, instruct patients and exposed individuals to clean the affected area [see Warnings and Precautions (5.1)].
- Wash hands and wear protective gloves when changing wound dressings [see Preparation (2.2)].
- Disinfect bandages from the first dressing change with a virucidal agent, such as 70% isopropyl alcohol, 6% hydrogen peroxide, or <0.4% ammonium chloride, and dispose of the disinfected bandages in a separate sealed plastic bag in household waste. Dispose of the subsequent used dressings and cleaning materials into a sealed plastic bag and dispose in household waste [see Administration (2.3)].
Manufactured by:
Krystal Biotech, Inc.
2100 Wharton Street, Suite 701
Pittsburgh, PA 15203
U.S. License No. 2301VYJUVEK® is a registered trademark of Krystal Biotech, Inc.
© 2025 Krystal Biotech, Inc. All rights reserved.
-
MEDICATION GUIDE
VYJUVEK (vye-JOO-vek) Gel
(beremagene geperpavec-svdt)
biological suspension mixed with excipient gel, for topical use
MEDICATION GUIDE
VYJUVEK (Vye-JOO-vek) Gel
(beremagene geperpavec-svdt)
biological suspension mixed with gel, for topical use
What is the most important information I should know about VYJUVEK Gel?
…The VYJUVEK® biological suspension must be mixed with the gel at the pharmacy prior to use.
…VYJUVEK gel should be applied by a healthcare professional (HCP) or by a patient/caregiver either at a healthcare professional setting (e.g., clinical) or a home setting.What is VYJUVEK Gel?
…VYJUVEK gel is a prescription medicine for the treatment of wounds (topical use only), in adult and pediatric patients who have dominant or recessive dystrophic epidermolysis bullosa.Who should not take VYJUVEK Gel?
… Individuals who are pregnant should not prepare or apply VYJUVEK gel and should avoid direct contact with the treated wounds or dressings from treated wounds.Before taking VYJUVEK Gel, tell your healthcare provider about all of your medical conditions, including if you:
… are pregnant or plan to become pregnant. It is not known if VYJUVEK gel will harm your unborn baby.
…are breast feeding or plan to breast feed. It is not known if VYJUVEK gel passes into your breast milk.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.How should I take VYJUVEK Gel?
…See the Instructions For Use for detailed instructions.
…Use VYJUVEK exactly as your healthcare providers tells you to and do not use past the expiration date. If you have questions, reach out to your provider or the pharmacy for further instructions.What should I avoid while taking VYJUVEK Gel?
…Avoid direct contact with treated wounds (e.g., touching or scratching) and dressings of treated wounds until the next dressing change following VYJUVEK gel application.
…Individuals should wear protective gloves when assisting with changing wound dressings and handling the disposal of the dressings.What are the possible side effects of VYJUVEK Gel?
…The most common side effects of VYJUVEK gel are itching, chills, redness, rash, cough, and runny nose.Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store VYJUVEK Gel?
…See the Instructions For Use for detailed instructions.
…VYJUVEK gel syringes may remain at room temperature (20° to 25°C (68° to 77°F)) for up to 8 hours prior to application. Administration syringes can be kept in an appropriate insulated or temperature regulated secondary container for up to 48 hours at 2° to 8°C (35.6° to 46.4°F).
…Follow expiration information provided by the pharmacy. If you have any questions, reach out to the pharmacy for additional information.General information about the safe and effective use of VYJUVEK Gel
…Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use VYJUVEK gel for a condition for which it was not prescribed. Do not give VYJUVEK gel to other people, even if they have the same symptoms that you have. It may harm them.
…You can ask your pharmacist or healthcare provider for information about VYJUVEK gel that is written for health professionals.What are the ingredients in VYJUVEK Gel?
Active ingredients: beremagene geperpavec-svdtInactive ingredients: glycerol, sodium chloride, sodium phosphate dibasic, potassium chloride, potassium phosphate monobasic, hydroxypropyl methylcellulose, and tromethamine
Manufactured by: Krystal Biotech, Inc.
2100 Wharton Street, Suite 701
Pittsburgh, PA 15203
U.S. License No. 2301
For more information, go to www.vyjuvek.com or call 1-844-5-KRYSTAL (1-844-5-579-7825)This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: 09/2025 -
INSTRUCTIONS FOR USE
VYJUVEK (vye-JOO-vek) Gel
(beremagene geperpavec-svdt)
biological suspension mixed with excipient gel, for topical use
This Instructions for Use contains information on how to apply VYJUVEK gel.
Read the Instructions for Use before you start using VYJUVEK gel. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
You or your caregiver should be shown how to apply VYJUVEK gel before the first use.
VyjuvekGel Syringe
Important Information You Need to Know Before Applying VYJUVEK Gel
Before Starting VYJUVEK GelIndividuals who are pregnant or nursing should not prepare or apply VYJUVEK gel and should avoid direct contact with the treated wounds or dressings from treated wounds, as this has not been formally studied. It is important to tell your healthcare provider if you are breastfeeding or plan to do so. They will then help you decide whether to stop breast-feeding, or whether to stop taking VYJUVEK gel, taking into account the benefit of breast-feeding to the baby and the benefit of VYJUVEK gel to you.
If you are allergic to beremagene geperpavec-svdt or any of its ingredients, you should not be given VYJUVEK gel.
Tell your healthcare provider about your standard of care routine including medications (prescribed and over the counter), bathing routine, dressing use, etc. before starting VYJUVEK gel.
How is VYJUVEK Gel Supplied?
Syringes containing VYJUVEK gel will be provided by the pharmacy in a sealable plastic bag. VYJUVEK gel in the syringes is ready for administration.Measures for Accidental Exposure
To avoid accidental exposure follow the below instructions for the administration and handling of VYJUVEK gel:- Wash hands and wear protective gloves while administering VYJUVEK gel.
- In the event of an accidental exposure through a splash to the eyes or mouth, flush with clean water for at least 15 minutes.
- Avoid direct contact with treated wounds (e.g., touching or scratching) and dressings of treated wounds until the next dressing change.
- Disinfect bandages from the first dressing change with a virucidal agent, such as 70% isopropyl alcohol, 6% hydrogen peroxide, or <0.4% ammonium chloride, and dispose of the disinfected bandages in a separate sealed plastic bag in household waste.
Storing VYJUVEK Gel Syringes
Follow the instructions provided by the pharmacy and do not use the syringes past their expiration.- VYJUVEK gel syringes are provided by the pharmacy in a refrigerated (2° to 8°C (35.6° to 46.4°F)) container.
- VYJUVEK gel syringes can be stored at room temperature (20 to 25°C (68° to 77°F)) for up to 8 hours prior to application. Administration syringes can be kept in an appropriate insulated or temperature regulated secondary container for up to 48 hours at 2° to 8°C (35.6° to 46.4°F).
- Store VYJUVEK gel syringes out of the reach of children.
- Do NOT heat the syringes or place them in the microwave.
Applying VYJUVEK Gel
Work with your healthcare provider to determine the best wound preparation, administration and dressing routine that works for you.It may not be possible to treat all wounds each week. VYJUVEK gel should be applied to wounds until they are closed before selecting new wound(s) to treat. Weekly treatment of previously treated wounds should be prioritized if they re-open.
If a dose is missed, VYJUVEK gel should be administered as soon as possible, and weekly treatments will continue. It is not recommended to stop your treatment without first consulting your doctor or nurse.
Wound Preparation
The wounds should be clean before applying VYJUVEK gel.- Gently remove any medicines, ointments, drainage, scabbing, and dead skin at the wound area.
-
- Bathing is not required.
- Leaving wounds uncovered can be painful to some patients. Ensure your area is set-up and ready prior to beginning application.
-
- In some cases, it may be best to keep wounds covered and treat one area at a time, removing dressings as you go.
- Do not use products or dressing that may contain anti-viral agents within 24 hours prior to use of VYJUVEK gel.
-
- If you are unsure if a product contains an anti-viral agent, refer to the Manufacturer’s website at the end of this document for more information or contact your healthcare provider on what to avoid.
Supplies
Below is the list of supplies needed for VYJUVEK gel administration:
- VYJUVEK gel syringes
- Hydrophobic dressing
- Refer to the Manufacturer’s website at the end of this document or contact your healthcare provider for more information on hydrophobic dressing options.
- Clean scissors
- Standard dressing
- Gloves
- Biohazard container
- Virucidal agent and absorbent material for clean-up
Follow the steps below for VYJUVEK gel administration.
The administration syringe should be primed prior to the initial application by pulling the plunger down and gently pushing it upwards, so that a small droplet of VYJUVEK gel forms at the tip of the syringe.
Step 1.Apply VYJUVEK gel to the selected wound(s) in droplets spaced evenly within the wound, approximately 1 cm-by-1 cm apart (width of a fingertip). The resulting droplet pattern should loosely resemble a grid. Avoid touching the administration syringe directly to the wound, only the droplet will touch the wound ( Figure 1).Note:You can treat the wound underneath the blister cap after it has been drained. Additionally, the VYJUVEK gel can be applied directly to the hydrophobic dressing in the same 1 cm-by-1 cm pattern to the size of the wound, if direct administration is difficult.
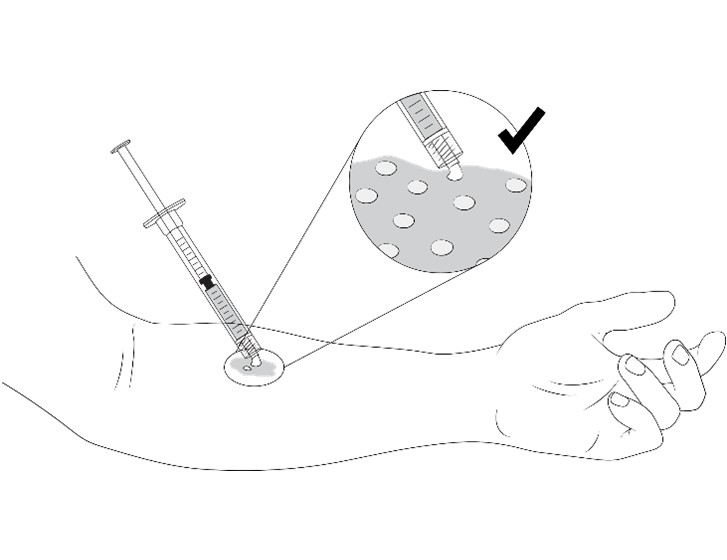
Figure 1 Application of VYJUVEK gel to the wound
Step 2.Use the scissors to cut the hydrophobic dressing to a size slightly larger than the wound and place the dressing atop the VYJUVEK gel droplets ( Figure 2).
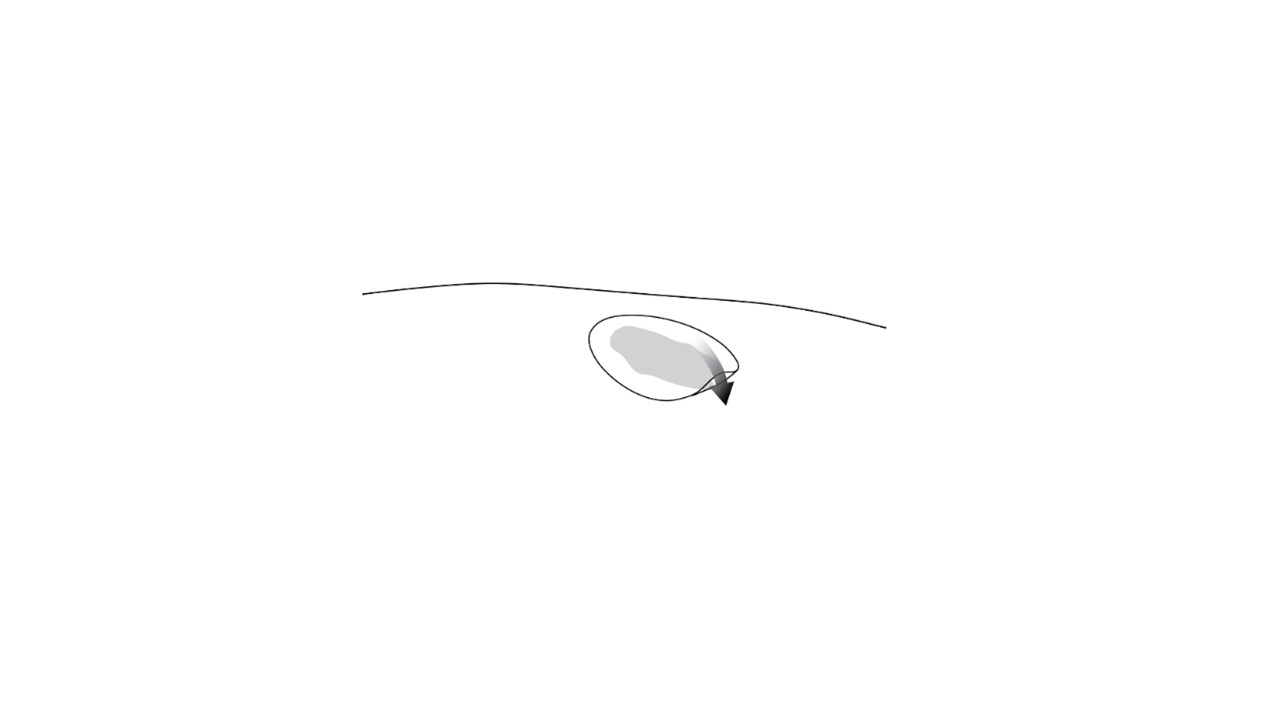
Figure 2 Placing the hydrophobic dressing over the treated wound
Step 3.Use the scissors to cut the standard dressing to a size slightly larger than the hydrophobic dressing and place the standard dressing atop the hydrophobic dressing ( Figure 3).
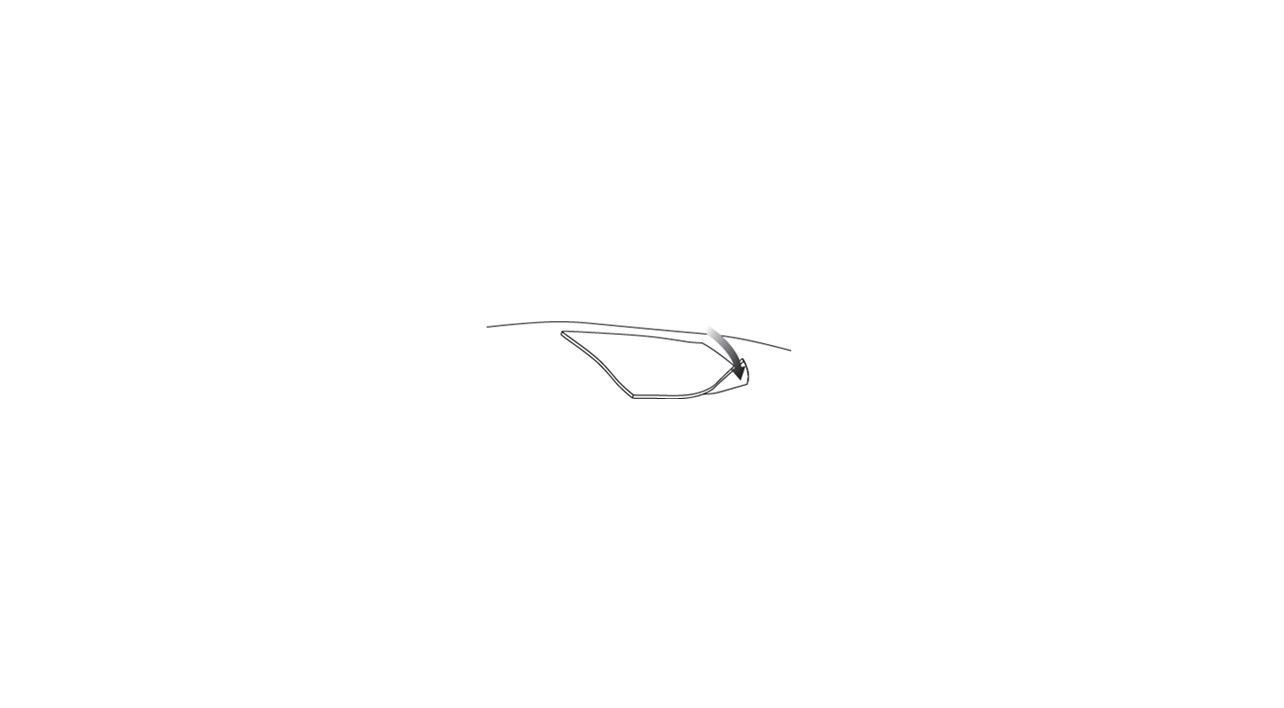
Figure 3 Placing the standard dressing over the hydrophobic dressing
Step 4.The VYJUVEK gel dressing should be left in place until the next dressing change. Disinfect VYJUVEK gel dressings, place in a sealable bag and discard into the household waste. Continue with your standard of care routine.
Disposal and Clean-up of VYJUVEK Gel
Clean all surfaces that may have come in contact with VYJUVEK gel and treat all spills (blot using absorbent material) with a virucidal agent such as 70% isopropyl alcohol, 6% hydrogen peroxide, or <0.4% ammonium chloride.Dispose all materials (e.g., used and unused syringe (expired), cleaning materials) that may have come in contact with VYJUVEK gel into a biohazard container.
Once the VYJUVEK gel dressings are removed and disinfected with a virucidal agent, place them in a sealable bag and discard into the household waste. Standard of care routine can then be continued.
Additional Information
Manufactured by: Krystal Biotech, Inc.
2100 Wharton Street, Suite 701
Pittsburgh, PA 15203
U.S. License No. 2301For more information, go to www.vyjuvek.com or call 1-844-5-KRYSTAL (1-844-5-579-7825)
This “Instructions for Use” has been approved by the U.S. Food and Drug Administration.
Approved: September 2025
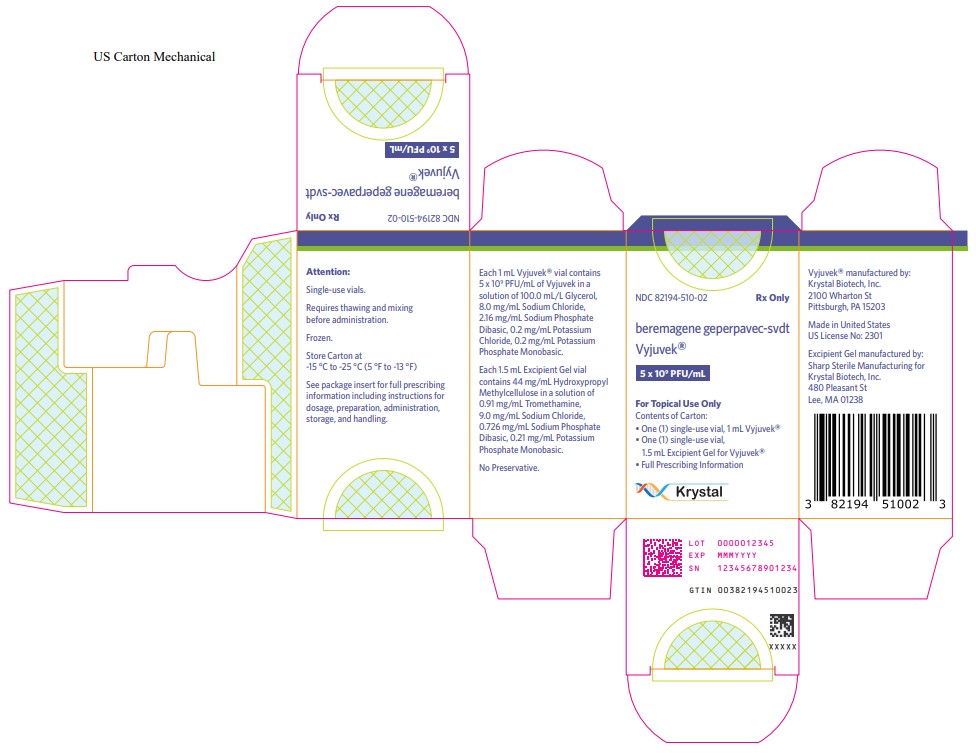
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
VYJUVEK
vyjuvek kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 82194-510 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 82194-510-02 1 in 1 CARTON; Type 0: Not a Combination Product 05/19/2023 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL 1 mL Part 2 1 VIAL 1.5 mL Part 1 of 2 VYJUVEK BIOLOGICAL SUSPENSION
beremagene geperpavec suspensionProduct Information Item Code (Source) NDC: 82194-501 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BEREMAGENE GEPERPAVEC (UNII: AQN7K24KQU) (BEREMAGENE GEPERPAVEC - UNII:AQN7K24KQU) BEREMAGENE GEPERPAVEC 5000000000 [PFU] in 1 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM PHOSPHATE DIBASIC DIHYDRATE (UNII: 94255I6E2T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 82194-501-01 1 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125774 Part 2 of 2 EXCIPIENT GEL
excipient gelProduct Information Item Code (Source) NDC: 82194-001 Route of Administration TOPICAL Inactive Ingredients Ingredient Name Strength HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) 1 mL in 1 mL TROMETHAMINE (UNII: 023C2WHX2V) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM PHOSPHATE DIBASIC DIHYDRATE (UNII: 94255I6E2T) POTASSIUM PHOSPHATE, MONOBASIC (UNII: 4J9FJ0HL51) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 82194-001-01 1.5 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125774 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125774 05/19/2023 Labeler - Krystal Biotech, Inc. (021814762) Establishment Name Address ID/FEI Business Operations Krystal Biotech, Inc. 021814762 manufacture(82194-510, 82194-501, 82194-001)
Trademark Results [VYJUVEK]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 VYJUVEK 90634232 not registered Live/Pending |
Krystal Biotech, Inc. 2021-04-09 |
 VYJUVEK 90634168 not registered Live/Pending |
Krystal Biotech, Inc. 2021-04-09 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.